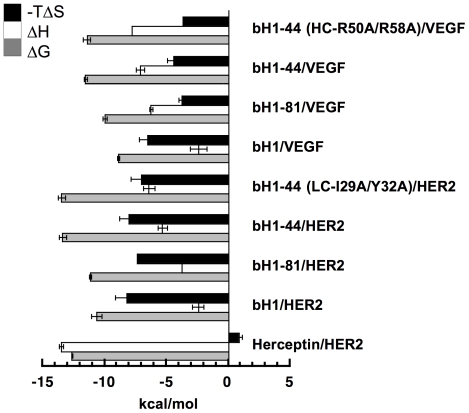
Figure 4. Thermodynamic profiles of the bH1 variants and Herceptin.
The entropic component (−TΔS) and enthalpic component (ΔH) of the binding free energy (ΔG) measured at 30°C in phosphate buffer (pH 7.4) are shown in kcal/mol. ΔG was derived from the dissociation constant (KD) measured with SPR (See Figure 2) (ΔG = RTlnKD), and ΔH was measured using ITC. −TΔS was calculated from the ΔG and ΔH according to −TΔS = ΔG−ΔH. Error bars of ΔG and ΔH represent standard deviation of three independent measurements (exception: the enthalpies of the bH1-81/HER2 and bH1-44(R50A/R58A)/VEGF interactions were measured only once, thus no error bar), and the errors of −TΔS are combined errors of ΔG and ΔH.