Abstract
Ionising radiation (IR) is a known carcinogen and poses a significant risk to the haematopoietic system for the development of leukaemia in part by induction of genomic instability. Induction of chronic oxidative stress has been assumed to play an important role in mediating the effect of IR on the haematopoietic system. However, there was no direct evidence to support this hypothesis prior to our studies. In our recent studies, we showed that exposure of mice to total body irradiation (TBI) induces persistent oxidative stress selectively in haematopoietic stem cells (HSCs) at least in part via up-regulation of nicotinamide adenine dinucleotide phosphate oxidase (NOX) 4. Now, we found that post-TBI treatment with diphenylene iodonium (DPI), a pan NOX inhibitor, not only significantly reduces TBI-induced increases in reactive oxygen species (ROS) production, oxidative DNA damage and DNA double-strand breaks in HSCs but also dramatically decreases the number of cells with unstable chromosomal aberrations in the clonal progeny of irradiated HSCs. The effects of DPI are comparable to Mn (III) meso-tetrakis (N-ethylpyridinium-2-yl) porphyrin, a superoxide dismutase mimetic and a potent antioxidant. These findings demonstrate that increased production of ROS by NOX in HSCs mediates the induction of haematopoietic genomic instability by IR and that NOX may represent a novel molecular target to inhibit TBI-induced genomic instability.
Introduction
It has been well established that exposure to ionising radiation (IR) increases non-clonal cytogenetic aberrations either in the clonal descendants of irradiated haematopoietic stem cells (HSCs) (referred as IR-induced genomic instability) or in the progeny of un-irradiated HSCs receiving signals from neighbouring irradiated cells (known as IR-induced bystander effect) (1,2). These non-targeted effects of IR represent a significant risk for the development of leukaemia and cancer (3). Recently, significant progresses have been made in understanding the underlying mechanisms of the bystander effect. It is likely mediated by IR-activated macrophages that can cause bystander oxidative damage to neighbouring un-irradiated HSCs via increased production of reactive oxygen species (ROS), reactive nitrogen species and various inflammatory cytokines (4). Similarly, it has been hypothesised that induction of chronic oxidative stress is also the primary cause of IR-induced genomic instability (2). Although this hypothesis is well supported by numerous in vitro observations in which various genetically unstable cells induced by IR exhibit increased production of ROS (5–7), there was no direct in vivo evidence to support this hypothesis until our recent study. In that study, we found that exposure of mice to total body irradiation (TBI) induces a sustained increase in ROS production selectively in HSCs (8). Compared to their progeny, HSCs are dormant and have fewer mitochondria (9,10) and express nicotinamide adenine dinucleotide phosphate oxidase (NOX) 1, 2 and 4 and various regulatory subunits (9,10). It was estimated that NOX-mediated extra-mitochondrial oxygen consumption accounts about half of the endogenous cell respiration in HSCs (9). After exposure to IR, HSCs from irradiated mice express increased levels of NOX4 (8). Pharmacological inhibition of NOX activity with diphenylene iodonium (DPI) (a pan NOX inhibitor), but not with a cyclooxygenases, lipoxygenases or mitochondrial complex I inhibitor, attenuated ROS production by irradiated HSCs, suggesting that NOX may be primarily responsible for IR-induced increase in ROS production in HSCs (8). These findings prompt us to examine if increased production of ROS by NOX mediates TBI-induced haematopoietic genomic instability and whether inhibition of ROS production by an NOX inhibitor such as DPI can attenuate the effect of TBI in comparison with Mn (III) meso-tetrakis (N-ethylpyridinium-2-yl) porphyrin (MnTE), a superoxide dismutase mimetic and a potent antioxidant.
Materials and methods
Reagents
Anti-Sca-1-phycoerythrin (PE) (clone E13-161.7, rat IgG2a), anti-c-kit-allophycocyanin (APC) (clone 2B8, rat IgG2b), biotin-conjugated anti-CD3e (clone 145-2C11, hamster IgG1), anti-CD45R/B220 (clone RA3-6B2, rat IgG2a), anti-Gr-1 (clone RB6-8C5, rat IgG2b), anti-CD11b (clone M1/70, rat IgG2b), and anti-Ter-119 (clone Ter-119, rat IgG2b); purified rat anti-CD16/CD32 (clone 2.4G2, Fcgamma (Fcγ) receptor blocker, rat IgG2b); and streptavidin-fluorescein were purchased from BD-Pharmingen (San Diego, CA, USA). Anti-phospho-histone H2AX (Ser139) (γH2AX; clone JBW301) and anti-8-hydroxy-2′-deoxyguanosine (8-OH-dG) (clone N45.1) were purchased from Millipore (Billerica, MA, USA) and Cosmo Bio Co., Ltd (Japan), respectively. Hoechst-33342 and DPI were obtained from Sigma–Aldrich (St. Louis, MO, USA). MnTE was synthesised and purified as previously described (11). 2′,7′-Dichlorofluorescin diacetate (DCFDA) was obtained from Invitrogen (Carlsbad, CA, USA).
Mice
Male C57BL/6 mice were purchased from Charles River Laboratories through the National Cancer Institute. Mice were housed four to a cage at the Medical University of South Carolina (MUSC) and University of Arkansas for Medical Sciences (UAMS) Association for Assessment and Accreditation of Laboratory Animal Care International-certified animal facilities. They received food and water ad libitum. All mice were used at approximately 8–12 weeks of age. The Institutional Animal Care and Use Committees of MUSC and UAMS approved all experimental procedures used in this study.
TBI and DPI and MnTE treatment
Mice were exposed to a sublethal dose (6.5 Gy) of IR in a JL Shepherd Model 143 caesium-137 γ-irradiator (JL Shepherd, Glendale, CA, USA) at a rate of 2.4 Gy/min. Mice were irradiated on a rotating platform. For MnTE treatment, 6 h after TBI, mice were treated with MnTE (6 mg/kg, 0.1 ml) by subcutaneous (s.c.) injection and then the injection was repeated every day (q.d.) for 30 days. For DPI treatment, mice were administered DPI (1 mg/kg, 0.1 ml) by s.c. injection every other day (q48h) at 6 h after TBI for 30 days. As a control, mice were irradiated but received s.c. injection of the same volume of vehicle [0.1 ml phosphate-buffered aline (PBS)].
Isolation of bone marrow mononuclear cells, lineage-negative haematopoietic cells and HSCs (Lin−c-kit+Sca1+)
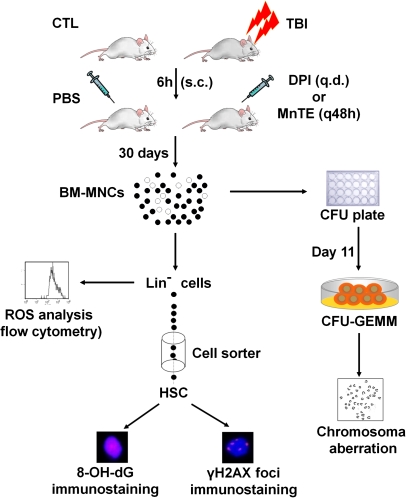
Bone marrow mononuclear cells (BM-MNCs) and lineage-negative (Lin−) cells were isolated as described previously (12). HSCs were sorted after pre-incubation of Lin− cells with anti-CD16/32 antibody to block the Fcγ receptors and then labelled with anti-Sca-1-PE and anti-c-kit-APC antibodies by an Aria cell sorter (Becton–Dickinson, San Jose, CA, USA) as illustrated in Figure 1.
Fig. 1.
Schematic illustration of the experimental design. All the analyses were done with the cells harvested from mice 30 days after exposure to 6.5 Gy TBI with s.c. injection of PBS (vehicle), DPI (1 mg/kg, q48h) or MnTE (6 mg/kg, q.d.) in comparison with the cells from un-irradiated control mice (CTL).
Analysis of intracellular ROS
Lin− cells (1 × 106/ml) stained with anti-Sca-1-PE and anti-c-kit-APC antibodies were suspended in PBS supplemented with 5 mM glucose, 1 mM CaCl2, 0.5 mM MgSO4, and 5 mg/ml bovine serum albumin and then incubated with DCFDA (10 μM) for 30 min at 37°C. The levels of ROS in HSCs were analysed by measuring the mean fluorescence intensity of 2′,7′-dichlorofluorescein using a FACSCalibur flow cytometer (Becton–Dickinson). For each sample, a minimum of 100 000–200 000 Lin− cells were acquired and the data were analysed using CellQuest software (Becton–Dickinson). In all experiments, PE and APC isotype controls and other positive and negative controls were included as appropriate.
Immunofluorescent 8-OH-dG and γH2AX staining
Immunofluorescent 8-OH-dG and γH2AX staining was done with anti-8-OH-dG (1:1000) or anti-γH2AX (1:1000) as we previously described (8). Approximately 200 nuclei images were acquired using a Zeiss Axio Observer.Z1 (Carl Zeiss, Oberkochen, Germany) with an Apo 60×/1.4 oil DICIII objective. The images were captured using AxioVision (4.7.1.0) software (Carl Zeiss).
Cytogenetic analyses
CFU-granulocyte/erythroid/macrophage/megakaryocyte (CFU-GEMM) colonies were developed by culturing BM-MNCs in MethoCult GF M3434 methylcellulose medium (Stem Cell Technologies, Vancouver, British Columbia, Canada). On day 11, cytogenetic preparations were obtained from CFU-GEMM individual colonies. Colcemid was added at 0.02 μg/ml and incubated at 37°C for 1 h. After wash, cells were suspended in pre-warmed hypotonic 0.075 M (0.55% w/v) of potassium chloride solution for 15 min at 37°C. Cells were then fixed by a fresh 3:1 methanol/acetic acid mixture. After three washes with fixative, cells were dropped onto cytogenetic slides in a humid chamber. Air-dried preparations were aged for overnight before Giemsa staining.
Statistical analysis
The data were analysed by analysis of variance (ANOVA). Differences among group means were analysed by Student-Newman-Keuls multiple comparisons test after one- or two-way ANOVA. For experiments in which only single experimental and control groups were used, group differences were examined by unpaired Student's t test. For analysis of unstable chromosomal aberrations, the data from three independent assays were pooled and the differences between the proportions of aberrant cells were analysed by Fisher's exact test. Differences were considered significant at P < 0.05. All of these analyses were done using GraphPad Prism (4.03) from GraphPad Software (San Diego, CA, USA).
Results and discussion
Maintenance of genomic stability has been shown to be crucial for the preservation of HSCs and for the prevention of leukaemia (13,14). However, HSCs conversely accrue more DNA damage than their progeny and are susceptible to the induction of genomic instability after exposure to IR. Induction of chronic oxidative stress has been hypothesised to mediate IR-induced haematopoietic genomic instability (2,5,6). This hypothesis is supported by our recent finding that exposure of mice to a sublethal dose of TBI induces a sustained increase in ROS production selectively in HSCs (8). Although an increased production of ROS by irradiated cells has been largely attributed to the dysfunction of mitochondria (5,6), cells can also produce ROS through activation and/or induction of NOX (15,16). ROS produced by NOX participate in regulation of many cell functions and also have been implicated in various pathological conditions induced by IR (15–17). In our previous study, we found that exposure of mice to TBI induces a sustained increase in ROS production selectively in HSCs in part by up-regulating the expression of NOX4 (8). In the present study, we examined if ROS produced by NOX play a causal role in the induction of haematopoietic genomic instability by IR and whether NOX can be targeted for intervention to reduce the non-targeted effect of IR on HSCs by the pan NOX inhibition DPI as well as MnTE, a superoxide dismutase mimetic and a potent antioxidant.
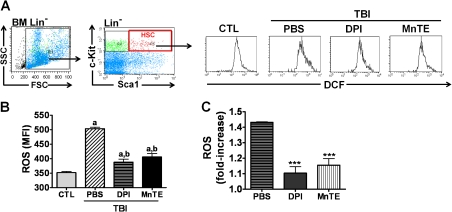
As shown in Figure 1, we exposed mice to a sublethal dose (6.5 Gy) of TBI. Six hours after irradiation, we administered DPI, MnTE, or vehicle (PBS) via s.c. injection to mice every other days for up to 30 days. HSCs were isolated from BM of different treatment groups and ROS levels were analysed by flow cytometry after a brief incubation with DCFDA (Figure 2A). The results from the analysis showed that there was a significant elevation of intracellular production of ROS (∼1.4-fold) in HSCs isolated from irradiated mice receiving vehicle treatment (Figure 2B and C). After DPI treatment, TBI-induced production of ROS was diminished in HSCs. Similarly, HSCs from irradiated mice treated with MnTE also displayed a significant reduction in TBI-induced ROS level. These findings confirm our recent observation that NOX is primarily responsible for IR-induced increase in ROS production in HSCs (8).
Fig. 2.
Administration of DPI or MnTE reduces TBI-induced persistent oxidative stress in HSCs. (A) A representative analysis of ROS production in HSCs by flow cytometry. (B) ROS production in HSCs as the mean fluorescent intensity of 2′,7′-dichlorofluorescein measured by flow cytometry. a, P < 0.05 versus un-irradiated control (CTL); and b, P < 0.05 versus TBI plus vehicle. (C) Fold-increase in ROS production from the cells harvested from mice 30 days after exposure to 6.5 Gy TBI with s.c. injection of PBS, DPI or MnTE compared to the cells from un-irradiated control mice. The data are presented as mean ± standard error from three independent experiments employing pooled cells from at least five mice per treatment group. ***P < 0.001 versus TBI plus vehicle.
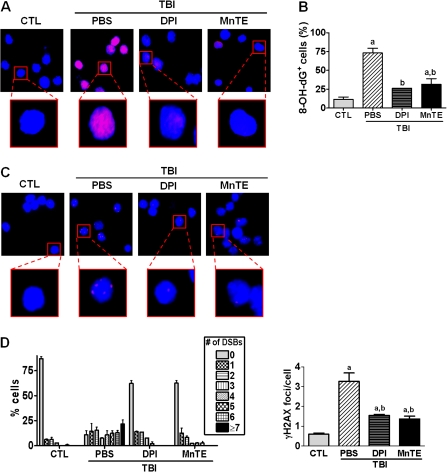
Next, we examined if the increased production of ROS by NOX mediates IR-induced persistent DNA damage in HSCs by immunostaining HSCs with antibodies against 8-OH-dG and γH2AX that detect oxidative DNA damage and DNA double-strand breaks (DSBs) (18,19), respectively. As shown in Figure 3, HSCs from un-irradiated mice exhibited minimal 8-OH-dG immunostaining and had very few γH2AX foci. Exposure to TBI resulted in significant increases in 8-OH-dG immunostaining and numbers of γH2AX foci in HSCs. The increases were effectively attenuated by the treatment with DPI. Similarly, a marked reduction in both 8-OH-dG immunostaining and numbers of γ-H2AX foci was found in HSCs from irradiated mice after the MnTE treatment. In fact, the effect of DPI was quite comparable to that of MnTE, indicating that both DPI and MnTE can effectively ameliorate TBI-induced oxidative DNA damage in HSCs.
Fig. 3.
Administration of DPI or MnTE reduces sustained oxidative DNA damage and DSBs in HSCs induced by TBI. Analysis of oxidative DNA damage in HSCs by 8-OH-dG immunostaining: (A) Representative photomicrographs of 8-OH-dG immunofluorescent staining (red) and nucleic counterstaining with Hoechst-33342 (blue); and (B) Percentage of cells with 8-OH-dG immunostaining. Analysis of DSBs in HSCs by γH2AX immunostaining: (C) Representative photomicrographs of γH2AX immunofluorescent staining (red) and nucleic counterstaining with Hoechst-33342 (blue); and (D) Percentage of cells with different numbers of γH2AX foci (left panel) and numbers of γH2AX foci per cell (right panel). The data are presented as mean ± standard error from three independent experiments with pooled cells from at least five mice per group. a, P < 0.05 versus un-irradiated control (CTL); and b, P < 0.05 versus TBI plus vehicle.
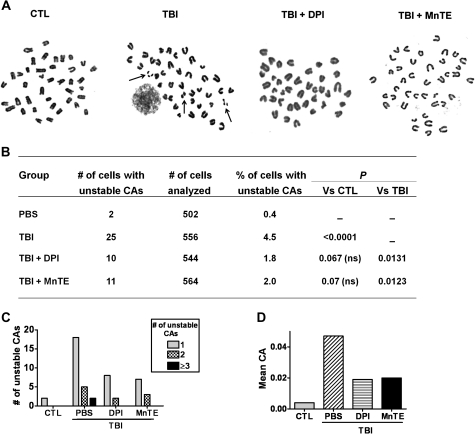
Finally, we investigated whether NOX-derived ROS play a causal role in IR-induced haematopoietic genomic instability by analysis of non-clonal cytogenetic changes in the clonal progeny of HSCs from irradiated mice with or without DPI treatment. As shown in Figure 4, the descendants of HSCs from un-irradiated mice had minimal numbers of unstable chromosomal aberrations (including chromatid breaks, chromosomal fragments, and minutes). After TBI, the numbers of unstable chromosomal aberrations were significantly increased. However, no clonal chromosomal aberrations (chromosomal translocations and deletions) were observed in the colonies of the irradiated cells that we examined, which is not unexpected considering the relatively low frequency of IR-induced stable clonal aberrations found in the previous studies (20). Intriguingly, after treatment with either DPI or MnTE, the numbers of unstable chromosomal aberrations were dramatically reduced. To the best of our knowledge, these findings provide the foremost direct evidence demonstrating that increase in ROS production plays a causal role in mediating IR-induced haematopoietic genomic instability. Furthermore, it is suggestive that NOX may represent a novel molecular target to inhibit TBI-induced genomic instability and NOX inhibitors and potent antioxidants such as MnTE may be used as effective chemopreventive agents to reduce IR-induced haematological malignancies.
Fig. 4.
Administration of DPI or MnTE attenuates TBI-induced haematopoietic genomic instability. (A) Representative photomicrographs of metaphase chromosome spreads from the clonal progeny of HSCs. Unstable chromosomal aberrations (CAs) are indicated by arrows. (B) CAs in the cells from the clonal progeny of HSCs. (C) Distribution of unstable CAs. (D) Mean of unstable CAs.
Funding
National Institutes of Health (R01-CA086688, CA102558, and AI080421); National Natural Science Foundation of China (NSFC 30828011); Winthrop W. Rockefeller Endowment for Leukaemia Research.
Acknowledgments
The authors thank Mrs Aimin Yang for her excellent technical assistance and Mr Richard Peppler for the flow cytometric analysis and cell sorting.
Conflict of interest statement: None declared.
References
- 1.Little JB. Radiation carcinogenesis. Carcinogenesis. 2000;21:397–404. doi: 10.1093/carcin/21.3.397. [DOI] [PubMed] [Google Scholar]
- 2.Wright EG, Coates PJ. Untargeted effects of ionizing radiation: implications for radiation pathology. Mutat. Res. 2006;597:119–132. doi: 10.1016/j.mrfmmm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 3.Huang L, Snyder AR, Morgan WF. Radiation-induced genomic instability and its implications for radiation carcinogenesis. Oncogene. 2003;22:5848–5854. doi: 10.1038/sj.onc.1206697. [DOI] [PubMed] [Google Scholar]
- 4.Lorimore SA, Chrystal JA, Robinson JI, Coates PJ, Wright EG. Chromosomal instability in unirradiated hematopoietic cells induced by macrophages exposed in vivo to ionizing radiation. Cancer Res. 2008;68:8122–8126. doi: 10.1158/0008-5472.CAN-08-0698. [DOI] [PubMed] [Google Scholar]
- 5.Kim GJ, Chandrasekaran K, Morgan WF. Mitochondrial dysfunction, persistently elevated levels of reactive oxygen species and radiation-induced genomic instability: a review. Mutagenesis. 2006;21:361–367. doi: 10.1093/mutage/gel048. [DOI] [PubMed] [Google Scholar]
- 6.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 7.Limoli CL, Giedzinski E. Induction of chromosomal instability by chronic oxidative stress. Neoplasia. 2003;5:339–346. doi: 10.1016/S1476-5586(03)80027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Liu L, Pazhanisamy SK, Li H, Meng A, Zhou D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic. Biol. Med. 2010;48:348–356. doi: 10.1016/j.freeradbiomed.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piccoli C, Ria R, Scrima R, Cela O, D'Aprile A, Boffoli D, Falzetti F, Tabilio A, Capitanio N. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J. Biol. Chem. 2005;280:26467–26476. doi: 10.1074/jbc.M500047200. [DOI] [PubMed] [Google Scholar]
- 10.Piccoli C, D'Aprile A, Ripoli M, Scrima R, Lecce L, Boffoli D, Tabilio A, Capitanio N. Bone-marrow derived hematopoietic stem/progenitor cells express multiple isoforms of NADPH oxidase and produce constitutively reactive oxygen species. Biochem. Biophys. Res. Commun. 2007;353:965–972. doi: 10.1016/j.bbrc.2006.12.148. [DOI] [PubMed] [Google Scholar]
- 11.Batinic-Haberle I, Benov L, Spasojevic I, Fridovich I. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J. Biol. Chem. 1998;273:24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–366. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenyon J, Gerson SL. The role of DNA damage repair in aging of adult stem cells. Nucleic Acids Res. 2007;35:7557–7565. doi: 10.1093/nar/gkm1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niedernhofer LJ. DNA repair is crucial for maintaining hematopoietic stem cell function. DNA Repair (Amst.) 2008;7:523–529. doi: 10.1016/j.dnarep.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 16.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins-Underwood JR, Zhao W, Sharpe JG, Robbins ME. NADPH oxidase mediates radiation-induced oxidative stress in rat brain microvascular endothelial cells. Free Radic. Biol. Med. 2008;45:929–938. doi: 10.1016/j.freeradbiomed.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toyokuni S, Iwasa Y, Kondo S, Tanaka T, Ochi H, Hiai H. Intranuclear distribution of 8-hydroxy-2'-deoxyguanosine. An immunocytochemical study. J. Histochem. Cytochem. 1999;47:833–836. doi: 10.1177/002215549904700613. [DOI] [PubMed] [Google Scholar]
- 20.Watson GE, Lorimore SA, Macdonald DA, Wright EG. Chromosomal instability in unirradiated cells induced in vivo by a bystander effect of ionizing radiation. Cancer Res. 2000;60:5608–5611. [PubMed] [Google Scholar]