Fig. 7. Model of MIA-induced IL-6 effects on the placenta.
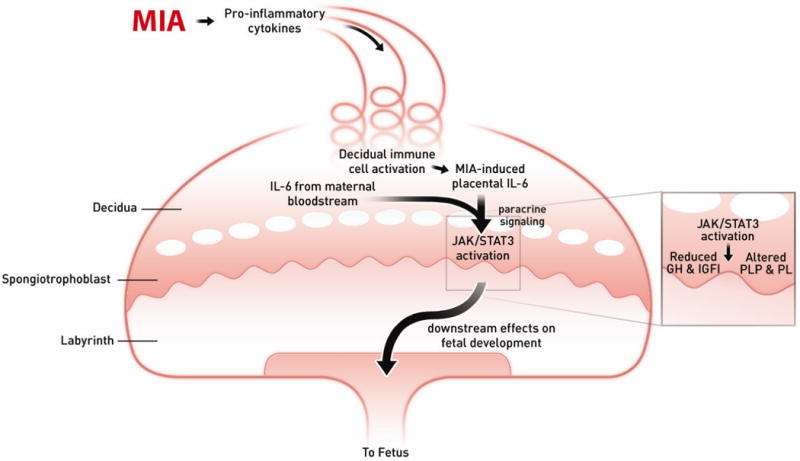
Maternal injection of poly(I:C) leads to the activation of the maternal immune system via a TLR3-mediated anti-viral response. Pro-inflammatory factors, including IL-6, are secreted by activated TLR3+ cells into the maternal bloodstream. As maternal blood circulates continuously through the placenta, IL-6 and soluble pro-inflammatory factors increase in the spiral arteries that descend through the decidua and spongiotrophoblast, as well as in the maternal blood spaces of the labyrinth, and circulate back up into the maternal compartment. Resident immune cells in the decidua are activated by maternal cytokines and other signaling factors to express CD69, and they propagate the inflammatory response by further cytokine release. IL-6 derived from decidual cells acts in a paracrine manner on target cells in the spongiotrophoblast layer. Ligation with the cognate receptor IL-6Ra and gp130 leads to signal transduction resulting in JAK/STAT3 activation and downstream changes in gene expression. Increases in acute phase proteins, such as SOCS3, down-regulate placental GH production and signaling. This leads to reduced IGFBP3 and IGFI. Global changes in STAT3 activation in the spongiotrophoblast layer alter the production of placenta-specific PLP and pro-lactin proteins. These changes in endocrine factors lead to acute placental pathophysiology and subsequent effects on fetal development.
