Abstract
The medial preoptic nucleus (MPN) and ventral bed nuclei of the stria terminalis (BST) are needed to maintain mating in sexually experienced male gerbils and rats. The gerbil ventral BST is also activated with mating, as assessed by Fos expression, as is the medial MPN (MPNm) of both species. In gerbils, many of those mating-activated cells contain glutamic acid decarboxylase (GAD), the enzyme that synthesizes γ-aminobutyric acid (GABA). Some of those are cells are projection neurons, but others may release GABA locally. Through actions in the medial preoptic area, GABA inhibits and testosterone (T) promotes male sex behavior. Thus, T may promote mating, in part, by decreasing GAD in MPNm or ventral BST cells. In rats, T increases GAD mRNA in the central MPN (MPNc), where MPN GABAergic cells are densest, but mating behavior does not change in sexually experienced males when the MPNc is ablated. Therefore, this study focused on the MPNm and ventral BST to ask whether their GABAergic cells respond to T or are sexually dimorphic. This was done by visualizing cells immunoreactive (IR) for GAD67, an isoform found primarily in cell bodies, in male and female gerbils and in castrated males with and without T. At both sites, males had more GAD67-IR cells than females, and T decreased GAD67-IR cell numbers in males. Thus, the MPNm and ventral BST have GABAergic cells that are sexually dimorphic and in which T decreases GAD, consistent with local effects of T and GABA on mating.
Keywords: glutamic acid decarboxylase
The medial preoptic nucleus (MPN) and ventral bed nuclei of the stria terminalis (BST) are needed to maintain mating in sexually experienced male rats and gerbils (Hansen et al., 1982; Everitt and Stacey, 1987; Yahr and Gregory, 1993; Finn and Yahr, 2005). The gerbil ventral BST is also activated with mating, as assessed by Fos expression, as is the medial MPN (MPNm) of both species (Baum and Everitt, 1992; Coolen et al., 1996; Heeb and Yahr, 1996). In gerbils, many of those mating-activated cells produce glutamic acid decarboxylase (GAD; Simmons and Yahr, 2003; Simmons et al., 2011), the enzyme that synthesizes γ-aminobutyric acid (GABA). Some of those cells are the source of a projection to the retrorubral field (RRF; Simmons et al., 2011) that affects mating in gerbils and rats (Finn and Yahr, 1993, 2005), but others may release GABA locally. Since testosterone (T) promotes (Hull et al., 2002) and GABA inhibits (Fernandez-Guasti et al., 1986a,b) male sex behavior via the medial preoptic area (MPOA), T may exert some of its effects by reducing GAD in the MPNm and ventral BST.
In a dense central core of GABAergic cells in the MPN that is probably the central MPN (MPNc; Swanson, 1992), T increases GAD65/67 mRNA levels in rats (Sagrillo and Selmanoff, 1997). MPN GAD cells are also densest in the gerbil MPNc (Simmons and Yahr, 2003). Given their density, MPNc GAD cells may dominate GABA-related assays of the MPN that include them. This could be especially true for effects of T since MPN androgen receptors are densest in the MPNc (Commins and Yahr, 1985; Simerly et al., 1990). The higher GABA turnover seen in the male versus female MPN in rats (Gratten and Selmanoff, 1997) may also reflect the fact that the MPNc is larger in males than females (Simerly et al., 1984; Bloch and Gorski, 1994).
However, effects in the MPNc are difficult to relate to the role of the MPN in mating in experienced males since their mating behavior does not change when the MPNc is destroyed (Arendash and Gorski, 1983; Yahr and Gregory, 1993). The MPNc may affect the development of sex behavior. Naïve male rats show temporary mating deficits after it is ablated (De Jonge et al. 1989), and in sheep, the MPNc, or sexually dimorphic nucleus, develops in parallel with partner preference, being smaller in rams that prefer rams as sex partners than in rams that prefer ewes (Roselli et al., 2004). In gerbils, MPNc volume correlates with the efficiency of mounts (i.e., the likelihood that they result in intromission), but not with mount rate (Yahr and Stephens, 1987). In contrast, the combined volume of the MPNm and ventral BST correlates with mount rate, not efficiency. Therefore, this study focused on GABAergic cells in the MPNm and ventral BST, which were visualized immunocytochemically in male and female gerbils and in castrated males with and without T using an antibody to GAD67, an isoform found primarily in cell bodies (Erlander et al., 1991).
MATERIALS AND METHODS
Animals
Gerbils purchased as adults (150-day-old males; 120-day-old females; Harlan Sprague-Dawley; Indianapolis, IN) were housed in same-sex pairs under a 14:10-hr light:dark cycle with food and water freely available. They were anesthetized with sodium pentobarbital before surgery (50 mg/kg) and perfusion (100 mg/kg). All procedures were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Surgery
Thirty males were randomly assigned to three groups. Two groups were castrated and implanted subcutaneously with 5-mm, Silastic capsules (3.2 mm o.d., 1.6 mm i.d.) that were empty or filled with T. Capsules this size produce physiological T levels (Clark et al., 1993) and maintain mating (Yahr et al., 1979) in castrates. The third group of males and ten females received sham operations. Animals were housed individually after surgery and perfused six weeks later. Surgeries and perfusions were staggered so subjects could be processed in five matched sets (two animals/group) to avoid confounding any staining variations related to tissue processing run with experimental conditions. Females' estrous states were not monitored.
Tissue fixation
Gerbils were perfused pericardially with saline and freshly prepared 4% paraformaldehyde (PFA; pH 7.3) in 0.1 M sodium phosphate buffer (PB). Brains were removed and postfixed for 1.5 hr at 4 °C in PFA and stored overnight at 4 °C in PB with 20% sucrose added for cryoprotection. The next day, they were frozen and cut coronally at 30 μm.
Immunocytochemistry
Unless noted, procedures were done at room temperature, rinses involved three changes of solution over 15–30 min, and both rinses and incubations involved gentle agitation. After a rinse in PB + 0.9% NaCl (PBS), sections were incubated for 1 hr in PBS + 10% normal goat serum (NGS) and then for 20 hr at 4 °C in PBS + 1% NGS + rabbit anti-GAD67 immunoglobulin G (IgG) K2 (AB108; Chemicon; Temecula, CA) at 1:2,000. After a PBS rinse, they were incubated for 1 hr in PBS + 1.5% NGS + biotinylated goat anti-rabbit IgG (Vector Labs; Burlingame, CA) at 1:200 before the antibodies were visualized with a Vectastain Elite kit (Vector Labs; Burlingame, CA) per the kit instructions. The chromogen, 3,3'-diaminobenzidine (Sigma, St. Louis, MO), was used at 0.05% in Tris-buffered saline (TBS; pH 7.6) and developed with 0.004% H2O2. Sections were rinsed and stored in PBS until mounted onto slides coated with gelatin/chrom-alum. After air drying, they were dehydrated in graded alcohols, delipidized with Histoclear (National Diagnostics; Atlanta, GA) and coverslipped using DePeX (BDH Laboratory Supplies; Poole, UK).
Histology
None of the sections was analyzed until all had been processed and mounted. That allowed slides to be coded so they could be examined without knowledge of a subject's sex or treatment. Sections were viewed at 500× under brightfield illumination using a blue glass filter. A gridded counting area was superimposed, parallel to the midline, with a drawing tube. It was square (170 μm/side) for the ventral BST and a vertically oriented rectangle (100 × 240 μm) for the MPNm. The latter was positioned caudally and ventromedial to where the MPNc lies in males. Both counting areas fit wholly within their cell groups.
In one section per side, immunoreactive (IR) cell bodies in the counting area were charted. IR cells were identified by diffuse brown reaction product in the soma in a size and shape appropriate for neuronal cell bodies. Ambiguous profiles were not charted. All chartings were done by the same observer, and all MPNm chartings were done before ventral BST chartings began, to ensure as much consistency as possible across groups. Cell counts were done from the chartings. Paired t-tests showed no difference in counts across sides at either site, so counts were averaged across sides for each cell group and subject.
Nomenclature
The gerbil MPNm and ventral BST were initially identified as the medial and lateral cell groups of a sexually dimorphic area (SDA) in the gerbil hypothalamus (Commins and Yahr, 1984a). Based on homologies discussed by Finn et al. (1993), the medial SDA, the area lateral to it and the SDA pars compacta correspond to the rat MPNm, lateral MPN and MPNc, respectively (Swanson, 1992). The lateral SDA corresponds (Finn and Yahr, 2005) to the magnocellular subnucleus of the BST plus the parts of the ventral subnucleus lying ventral and lateral to it in rats (Ju and Swanson, 1989).
Statistical analysis
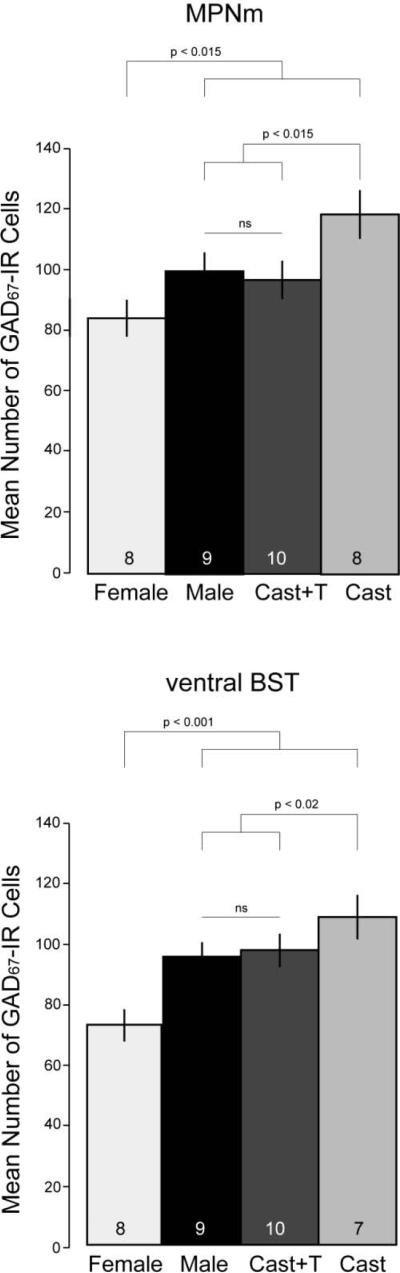
Data were analyzed with a 4 (groups) by 5 (processing set) analysis of variance followed by orthogonal contrasts comparing females to all males, males exposed to T (endogenous or exogenous) to males lacking T, and gonadally intact males to castrates given T. This was done with Systat v. 8 software (Chicago, IL). One subject died, tissue from four others was damaged, and ventral BST staining was blotchy in another, making it unusable. The final N/group is shown in Figure 1.
Fig. 1.
Sex differences and effects of testosterone (T) on cells immunoreactive (IR) for glutamic acid decarboxlyase67 (GAD67) in the medial part of the medial preoptic nucleus (MPNm) and ventral bed nuclei of the stria terminalis (ventral BST) of gerbils. Data are shown as group means ± SEM. The N is given at the bottom of each bar. Cell counts are for areas of 0.024 mm2 for the MPNm and 0.029 mm2 for the ventral BST. Cast = castrated male.
Image preparation
Digitized images were acquired with a Zeiss AxioImager M2 light microscope using a 10× objective and AxioVision software v4.7 and were combined in Adobe Photoshop CS5. To better reproduce the appearance of the tissue at microscopy, the image was adjusted for tonal qualities using the levels tool.
RESULTS
The groups differed overall in the number of GAD67-IR cells seen in the MPNm [F(3,15) = 5.12, p < 0.015] and ventral BST [F(3,14) = 11.95, p < 0.001]. As shown in Figure 1, males had more GAD67-IR cells than females at both sites [MPNm: (F(1,15) = 7.64, p < 0.015; ventral BST: F(1,14) = 30.84, p < 0.001]. Relative to females, intact males had 19% more GAD67-IR cells in the MPNm and 33% more in the ventral BST.
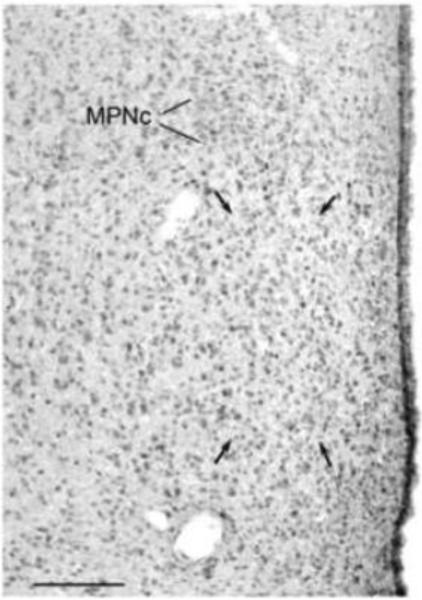
Among males, castrates with empty implants had more GAD67-IR cells at each site than intact males or castrates given T [MPNm: F(1,15) = 8.19, p < 0.015; ventral BST: F(1,14) = 7.28, p < 0.02]. Relative to intact males, castrates not given T had 19% more GAD67-IR cells in the MPNm and 14% more in the ventral BST. The number of GAD67-IR cells did not differ at either site between T-treated castrates and intact males. GAD67 staining in the MPNm is illustrated in Figure 2 (for GAD67-IR ventral BST cells, see Simmons et al., 2011).
Fig. 2.
Montage of photomicrographs of a coronal section through the medial preoptic nucleus (MPN) of a castrated male gerbil showing cells IR for GAD67. Arrows point to the corners of a cell-count area in the medial MPN. Bar = 0.1 mm. MPNc = central MPN.
Differences related to tissue processing set were significant for the ventral BST [F(4,14) = 6.23, p < 0.005] but not the MPNm. The effects of processing set did not interact with the effects of treatment at either site.
DISCUSSION
Visualizing MPNm and ventral BST cells that contain GAD67 showed that they are either more abundant in male than female gerbils, contain enough more GAD67 in males for more of them to be seen, or both. In males, fewer GAD cells were seen in the presence than in the absence of T. GAD levels per cell probably account for these effects of T since it seems unlikely that the number of neurons would increase 14–19% in six weeks in mature adults, though larger changes in cell genesis occur in the rat MPN at adolescence (Ahmed et al., 2008).
The sex difference in GABAergic cell density in the gerbil MPNm (outside the MPNc) is in the same direction (males > females) as the sex difference seen in GABA turnover in rat MPN samples that included the MPNc (Grattan and Selmanoff, 1997), though the latter dimorphism was much larger. That larger dimorphism may reflect inclusion of the MPNc, which has a very high density of GABAergic cells (Sagrillo and Selmanoff, 1997; Simmons and Yahr, 2003) and is larger in males than females (Simerly et al., 1984; Bloch and Gorski, 1994).
The decrease in GAD induced by T in the MPNm and ventral BST suggests that the T-induced increase in GAD mRNA seen in the rat MPN (Sagrillo and Selmanoff, 1997) may have occurred largely in the MPNc, obscuring opposite effects in the MPNm. Similarly, the GAD decreases seen here may have obscured opposite effects of T on some GABAergic cells in the MPNm and ventral BST. For example, both areas contain cells that promote mating in gerbils and rats via a projection to the RRF (Finn and Yahr, 1994, 2005) that is GABAergic in gerbils (Simmons et al., 2011). If T promotes mating by regulating their GAD, it should increase it.
Interestingly, both sites where T decreases GAD in male gerbils are sites where it stimulates another transmitter-related enzyme, acetylcholinesterase (AChE; Commins and Yahr, 1984b). Since MPNm and ventral BST cells lack the enzyme needed to synthesize acetylcholine (ACh; Kimura et al., 1981; Armstrong et al., 1983; Commins and Yahr, 1984a), their AChE cells are presumably cholinoceptive. Unlike other cell types identified in these areas, cholinoceptive and GABAergic cells appear nearly as dense as Nissl-stained cells (Commins and Yahr, 1984a,b; Simmons and Yahr, 2003, 2011a,b; Simmons et al., 2011). Thus, it seems likely that some cells there are both GABAergic and cholinoceptive. If T decreases GAD and increases AChE in the same cells, their reduced release of GABA in response to stimulatory inputs under the influence of T would be amplified for cholinergic inputs due to faster degradation of incoming ACh.
Finally, while most MPNm (56%) and ventral BST cells (89%) activated with mating are GABAergic (Simmons and Yahr, 2003; Simmons et al., 2011), most GABAergic cells in these areas (73% MPNm; 85% ventral BST) are not activated with mating. Cells that promote a behavior by releasing less transmitter may not be expected to be activated when the behavior is displayed. Thus, GABAergic MPNm and ventral BST cells in which T decreases GAD, consistent with T promoting and GABA inhibiting mating via local actions, may be a different set of GABAergic cells than those that are activated with mating. The latter may affect the pace of mating or the timing of ejaculation.
ACKNOWLEDGMENTS
This work was supported by NIMH Research Grant MH-26481. Ms. Grace Lee provided technical assistance with surgeries and histology. Dr. D. A. Simmons did the photomicroscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, Don Carlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nature Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendash GW, Gorski RA. Effects of discrete lesions of the sexually dimorphic nucleus of the preoptic area or other medial preoptic regions on the sexual behavior of male rats. Brain Res. Bull. 1983;10:147–154. doi: 10.1016/0361-9230(83)90086-2. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in the rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J. Comp. Neurol. 1983;216:53–68. doi: 10.1002/cne.902160106. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Everitt BJ. Increased expression of c-fos in the medial preoptic area after mating in male rats: role of afferent inputs from the medial amygdala and midbrain central tegmental field. Neurosci. 1992;50:627–646. doi: 10.1016/0306-4522(92)90452-8. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Gorski RA. Cytoarchitectonic analysis of the SDN-POA of the intact and gonadectomized rat. J. Comp. Neurol. 1988;275:604–612. doi: 10.1002/cne.902750408. [DOI] [PubMed] [Google Scholar]
- Clark MM, Bishop AM, vom Saal FS, Galef BG. Responsiveness to testosterone of male gerbils from known intrauterine positions. Physiol. Behav. 1993;53:1183–1187. doi: 10.1016/0031-9384(93)90377-r. [DOI] [PubMed] [Google Scholar]
- Commins D, Yahr P. Adult testosterone levels influence the morphology of a sexually dimorphic area in the Mongolian gerbil brain. J. Comp. Neurol. 1984a;224:132–140. doi: 10.1002/cne.902240112. [DOI] [PubMed] [Google Scholar]
- Commins D, Yahr P. Acetylcholinesterase activity in the sexually dimorphic area of the gerbil brain: Sex differences and influences of adult gonadal steroids. J. Comp. Neurol. 1984b;224:123–131. doi: 10.1002/cne.902240111. [DOI] [PubMed] [Google Scholar]
- Commins D, Yahr P. Autoradiographic localization of estrogen and androgen receptors in the sexually dimorphic area and other regions of the gerbil brain. J. Comp. Neurol. 1985;231:473–489. doi: 10.1002/cne.902310406. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Peters HJPW, Veening JG. Fos-immunoreactivity in the rat brain following consummatory elements of sexual behavior: A sex comparison. Brain Res. 1996;738:67–82. doi: 10.1016/0006-8993(96)00763-9. [DOI] [PubMed] [Google Scholar]
- De Jonge FH, Louwerse AL, Ooms MP, Evers P, Endert E, Van De Poll NE. Lesions of the SDN-POA inhibit sexual behavior of male Wistar rats. Brain Res. Bull. 1989;23:483–492. doi: 10.1016/0361-9230(89)90194-9. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJK, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Stacey P. Studies of instrumental behavior with sexual reinforcement in male rats (Rattus norvegicus). II. Effects of preoptic area lesions, castration, and testosterone. J. Comp. Psychol. 1987;101:407–419. [PubMed] [Google Scholar]
- Fernández-Guasti A, Larsson K, Beyer C. GABAergic control of masculine sexual behavior. Pharmacol. Biochem. Behav. 1986a;24:1065–1070. doi: 10.1016/0091-3057(86)90456-9. [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Larsson K, Beyer C. Effect of bicuculline on sexual activity in castrated male rats. Physiol. Behav. 1986b;36:235–237. doi: 10.1016/0031-9384(86)90009-0. [DOI] [PubMed] [Google Scholar]
- Finn PD, De Vries GJ, Yahr P. Efferent projections of the sexually dimorphic area of the gerbil hypothalamus: anterograde identification and retrograde verification in males and females. J. Comp. Neurol. 1993;338:491–520. doi: 10.1002/cne.903380403. [DOI] [PubMed] [Google Scholar]
- Finn PD, Yahr P. Projection of the sexually dimorphic area of the gerbil hypothalamus to the retrorubral field is essential for male sexual behavior: Role of A8 and other cells. Behav. Neurosci. 1994;108:362–378. doi: 10.1037//0735-7044.108.2.362. [DOI] [PubMed] [Google Scholar]
- Finn PD, Yahr P. Projection from the ventral bed nucleus of the stria terminalis to the retrorubral field in rats and the effects of cells in these areas on mating in male rats versus gerbils. Horm. Behav. 2005;47:123–138. doi: 10.1016/j.yhbeh.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Selmanoff M. Sex differences in the activity of γ-aminobutyric acidergic neurons in the rat hypothalamus. Brain Res. 1997;775:244–249. doi: 10.1016/s0006-8993(97)01069-x. [DOI] [PubMed] [Google Scholar]
- Hansen S, Kohler Ch., Goldstein M, Steinbusch HVM. Effects of ibotenic acid-induced neuronal degeneration in the medial preoptic area and the lateral hypothalamic area on sexual behavior in the male rat. Brain Res. 1982;239:213–232. doi: 10.1016/0006-8993(82)90843-5. [DOI] [PubMed] [Google Scholar]
- Heeb MM, Yahr P. C-fos immunoreactivity in the sexually dimorphic area of the hypothalamus and related brain regions of male gerbils after exposure to sex-related stimuli or performance of specific sexual behaviors. Neurosci. 1996;72:1049–1071. doi: 10.1016/0306-4522(95)00602-8. [DOI] [PubMed] [Google Scholar]
- Hull EM, Meisel RL, Sachs BD. Male sexual behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press; San Diego, CA: 2002. pp. 3–137. [Google Scholar]
- Ju G, Swanson LW. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. Cytoarchitecture. J Comp Neurol. 1989;280:587–602. doi: 10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- Kimura H, McGeer PL, Peng JH, McGeer EG. The central cholinergic system studied by choline acetyltransferase immunohistochemistry in the cat. J. Comp. Neurol. 1981;200:151–201. doi: 10.1002/cne.902000202. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinol. 2004;145:478–483. doi: 10.1210/en.2003-1098. [DOI] [PubMed] [Google Scholar]
- Sagrillo CA, Selmanoff M. Castration decreases single cell levels of mRNA encoding glutamic acid decarboxylase in the diagonal band of Broca and the sexually dimorphic nucleus of the preoptic area. J. Neuroendocrinol. 1997;9:699–706. doi: 10.1046/j.1365-2826.1997.00630.x. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J. Comp. Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Gorski RA. Demonstration of a sexual dimorphism in the distribution of serotonin-immunoreactive fibers in the medial preoptic nucleus of the rat. J. Comp. Neurol. 1984;225:151–166. doi: 10.1002/cne.902250202. [DOI] [PubMed] [Google Scholar]
- Simmons DA, Hoffman NW, Yahr P. A forebrain-retrorubral pathway involved in male sex behavior is GABAergic and activated with mating in gerbils. Neurosci. 2011 doi: 10.1016/j.neuroscience.2010.11.048. in press. doi: 10.1016/j.neuroscience.2010.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DA, Yahr P. GABA and glutamate in mating-activated cells in the preoptic area and medial amygdala of male gerbils. J. Comp. Neurol. 2003;459:290–300. doi: 10.1002/cne.10605. [DOI] [PubMed] [Google Scholar]
- Simmons DA, Yahr P. Distribution of catecholaminergic and peptidergic cells in the gerbil medial amygdala, caudal preoptic area and caudal bed nucleus of the stria terminalis with a focus on areas activated at ejaculation. J. Chem. Neuroanat. 2011a;41:13–19. doi: 10.1016/j.jchemneu.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DA, Yahr P. Nitric oxidergic cells related to ejaculation in gerbil forebrain contain androgen receptors and respond to testosterone. J. Comp. Neurol. 2011b doi: 10.1002/cne.22557. in press. doi: 10.1002/cne.22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Elsevier; Amsterdam: 1992. [Google Scholar]
- Yahr P, Gregory JE. The medial and lateral cell groups of the sexually dimorphic area of the gerbil hypothalamus are essential for male sex behavior and act via separate pathways. Brain Res. 1993;631:287–296. doi: 10.1016/0006-8993(93)91547-6. [DOI] [PubMed] [Google Scholar]
- Yahr P, Newman A, Stephens DR. Sexual behavior and scent marking in male gerbils: Comparison of changes after castration and testosterone replacement. Horm. Behav. 1979;13:174–184. doi: 10.1016/0018-506x(79)90056-4. [DOI] [PubMed] [Google Scholar]
- Yahr P, Stephens DR. Hormonal control of sexual and scent marking behaviors of male gerbils in relation to the sexually dimorphic area of the hypothalamus. Horm. Behav. 1987;21:331–346. doi: 10.1016/0018-506x(87)90018-3. [DOI] [PubMed] [Google Scholar]