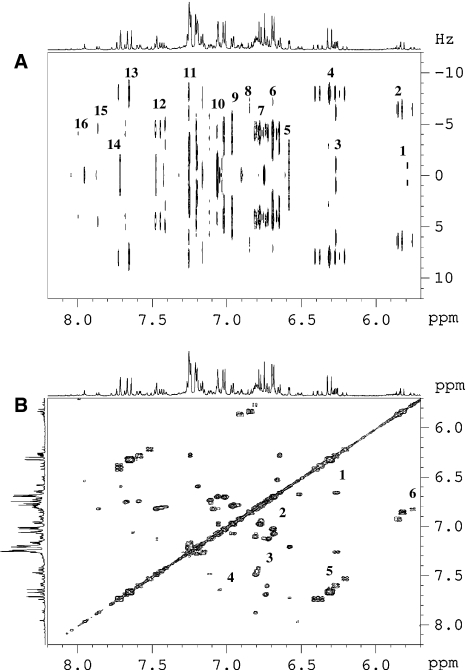
Fig. 2.
Two dimensional 1H–1H J-resolved (a) and 1H–1H COSY (b) spectra of ‘Riesling’ wine in the range of δ 5.5–δ 8.5. J-resolved (a) shows 1: H-6 of flavan-3-ols, 2: H-8 of cis-phenylpropanoids, 3: H-6 of flavonols, 4: H-8 of trans-phenylpropanoids, 5: H-2 & H-6 of cis-resveratrol, 6: H-8 of cis-resveratrol, 7: H-5 of phenylpropanoids, 8: H-7 of cis-phenylpropanoids, 9 & 10: H-6 of phenylpropanoids, 11: 1H of phenylalanine, 12: H-6 of p-coumaric acid, 13: H-7 trans-phenylpropanoids, 14: H-2 of flavonols, 15: H-2 & H-6 of p-benzoic acid, 16: H-2 of kaempferol. COSY (b) shows correlations between 1: H-6 and H-8 of quercetin, 2: H-5 and H-6 of phenylpropanoids, and H-7 and H-8 of resveratrol, 3: H-5 and H-6 of p-coumaric acid, 4: H-5 and H-6 of quercetin, 5: H-7 and H-8 of trans-phenylpropanoids, 6: H-7 and H-8 of cis-phenylpropanoids