Abstract
Objective
Patients’ beliefs about the causes of their illness have been associated with emotional adjustment and behavioral outcomes in several medical conditions; however, few studies have examined illness attributions among patients with COPD. In the current study, patterns of patients’ causal attributions for COPD were identified and examined in relation to health behaviors and symptoms.
Method
Three-hundred and ninety-four patients with COPD and ≥10 pack year history of smoking completed a self-report questionnaire that included the Illness Perception Questionnaire- Revised (IPQ-R).
Results
A factor analysis of the IPQ-R cause items using principal axis factoring yielded four individual items (i.e., smoking, heredity, pollution, and personal behavior) and one large factor that was primarily driven by psychological attributions. Ninety-three percent of patients agreed or strongly agreed that smoking was a cause of their COPD. Higher scores on the large IPQ-R factor were associated with reduced quality of life (r=.25, p<.001) and symptoms of anxiety (r=.33, p<.001) and depression (r=.31, p<.001), indicating that patients who attributed their COPD to psychological factors were more likely to have poorer emotional adjustment and quality of life.
Conclusions
Our finding of one large factor with several stand-alone items is in contrast with previous research that has derived a multi-factor structure for the cause items of the IPQ-R in other chronic illness populations. This difference may be due to the importance of smoking, environmental exposures, and heredity in the development of COPD. Future research should expand upon these specific attributions in COPD‥
Keywords: Chronic Obstructive Pulmonary Disease (COPD), Causal Attributions, Illness Perception Questionnaire- Revised (IPQ-R), Illness Beliefs
Introduction
There is substantial support in the behavioral medicine literature for the notion that patients’ responses to their illness are influenced by their own beliefs and cognitive model of their condition. The self-regulatory model of illness1, 2 proposes that patients form cognitive and emotional representations of their illness based on their previous experiences with the illness, information from health care providers, personal observations, and information from peers, family, and friends. These representations shape how patients cope with their illness, including their health behaviors and emotional reactions. Leventhal proposed five components of the illness representation including: identity, consequences, timeline, control/curability, and cause. This model has been tested in multiple chronic medical conditions (e.g., coronary artery disease3, rheumatoid arthritis4, 5, multiple sclerosis6, chronic fatigue syndrome7), and used to examine patients’ responses to acute events (e.g., myocardial infarction8, 9). Illness attributions have been associated with depressive symptoms, quality of life, medication adherence, and help seeking behavior.8
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of combined morbidity, disability, and mortality in the United States.10 COPD is characterized by a progressive decline in lung function. Airflow obstruction in COPD is not fully reversible, necessitating a focus on treating symptoms to reduce disability and improve patients’ quality of life. Management of COPD relies heavily on patients’ perceptions of their symptoms both in terms of initiating treatment of acute exacerbations, and in maintaining daily activity. Illness beliefs and psychological coping in COPD have been shown to be important for disability and clinical outcomes above and beyond objective physical measures of disease severity such as pulmonary function.11, 12 Indeed, a recent review of the literature13 called for incorporating illness perceptions into clinical care for patients with COPD to target maladaptive perceptions.
The major risk factor for COPD in the United States is cigarette smoking, with 80 – 90% of the risk of COPD attributable to smoking.14, 15 Given that a personal health behavior (i.e., smoking) is the primary factor contributing to COPD for current and former smokers, attributions about the cause of COPD may be a particularly important aspect of patients’ approach to managing their illness. For example, individuals who attribute COPD to their own behavior may be more likely to engage in healthy behaviors (such as quitting smoking and engaging in regular physical activity) after they are diagnosed with COPD.
The most commonly used measure of illness perceptions is the Illness Perception Questionnaire (IPQ).16, 17 The IPQ was initially developed in 1996 by Weinman and colleagues to quantitatively assess the five components of the illness representation model described above (i.e., identity, time-line, cure control, consequences, and cause).16 The IPQ was revised by Moss-Morris and colleagues in 2002 to address psychometric concerns and extend the range of causal items to include smoking, stress or worry, heredity, and chance or bad luck. Based on a factor analysis of the new IPQ-R causal items in a combined sample of patients with eight illnesses, the authors17 concluded that there were four primary factors (i.e., psychological attributions, risk factors, immunity, and chance). The authors of the IPQ-R noted that the subscales will vary in their applicability to different patient groups given that patients with different illnesses may have different primary attributions for the cause of their illness. As initially suggested by the IPQ authors, researchers are encouraged to adapt the scale to their particular illness and setting and to begin with a factor analysis to group items in samples of over 85. One study by Scharloo and colleagues18 has examined the psychometric structure of the IPQ-R causal items in a sample of 171 COPD patients in the Netherlands. The authors found four factors, but two had such low internal reliability that they were discarded. The two usable factors reflected behavioral and psychological attributions.
The primary aim of the current study was to extend prior research by examining the extent to which individuals with COPD and a significant smoking history endorse a variety of potential causes of their illness on the IPQ-R and to examine the factor structure of causal attributions on the IRQ-R. We chose a priori to focus our analysis on patients who have a significant smoking history (defined as >10 pack years) because these patients are likely to differ from patients without a significant smoking history with regard to beliefs about the causes of their illness. The secondary aim was to examine the association of causal attributions with health behaviors such as smoking and symptoms of breathlessness, depression, anxiety, and quality of life. We expected that we would replicate the four factor structure for IPQ-R cause items that has been derived in previous studies (i.e., psychological attributions, risk factors, immunity, and chance).17 We also anticipated that greater attributions for behavioral causes for COPD would be associated with better current health behaviors.
Method
Procedures
This study was approved by the Institutional Review Board at National Jewish Health and the Colorado Multiple Institutional Review Board. Individuals who were assessed or treated for COPD at two medical centers in Denver were included in this study. These two sites included a tertiary-care respiratory hospital and a university-affiliated public hospital, which were chosen to ensure a diverse sample. At the tertiary-care hospital, questionnaires were mailed to individuals with physician-diagnosed COPD who had given permission to be contacted regarding research studies. These individuals included clinic patients and patients who registered with the clinical research unit. At the public hospital, questionnaires were mailed to all individuals who were being treated for COPD at three internal medicine clinics, based on ICD-9 codes indicative of COPD.
Data were collected via an anonymous self-report questionnaire that was mailed between October 2005 and March 2007. Questionnaires were mailed to 1040 people and were returned by 542 people, a 52% response rate. Eleven respondents were excluded from analyses because they indicated that they did not have COPD. These respondents did not agree with their diagnosis and were excluded because many of the items on the questionnaire are applicable only to individuals who self-identify as having COPD. To improve our ability to assess the extent to which current or ex-smokers identify smoking as a cause of their COPD, we limited our sample to individuals with at least a 10 pack-year history of smoking. This resulted in excluding 59 individuals. We also required that participants have completed every item used to assess causal attributions for COPD, which resulted in excluding an additional 78 individuals. Closer examination of the missing data did not reveal a consistent pattern among the missing items. Over 95% of the sample completed 18 or more of the 20 items, and the skipped items were distributed approximately evenly across the measure. The final sample was 394.
Measures
Attributions about Cause of Illness
Beliefs about cause of illness were measured using the Cause subscale of the revised Illness Perception Questionnaire (IPQ-R).17 The Cause subscale has 18 items, including an item for smoking. Two additional items were added for this study (exercise habits/activity patterns, and infection or pneumonia). These items were added to capture additional potential causes that respondents were likely to endorse. For each item, respondents indicate the extent to which they agree or disagree that the item caused their COPD on a 5 point Likert scale, with 1 indicating “strong disagreement” and 5 indicating “strong agreement.” Response categories for each item are: strongly disagree, disagree, neither agree nor disagree, agree, and strongly agree.
Demographic and Clinical Characteristics
Table 1 shows demographic and clinical characteristics of the sample. For number of comorbid conditions, respondents indicated whether they had the following health problems: hypertension/high blood pressure, heart disease or heart surgery, diabetes or blood sugar problems, arthritis, bone problems, cancer, and other. The number of positive responses was summed to calculate the number of comorbid conditions. The number of medications prescribed for COPD was summed, with combination inhalers counted as two medications.
Table 1.
Demographic and Clinical Characteristics of the Sample
| N (%) | Variable |
|---|---|
| Gender | |
| 212 (53.9) | Male |
| 181 (46.1) | Female |
| Age | |
| 86 (21.8) | Less than 60 |
| 74 (18.8) | 60 to 64 |
| 92 (23.4) | 65 to 69 |
| 71 (18.0) | 70 to 74 |
| 71 (18.0) | 75 or more |
| Race/ethnicity | |
| 352 (89.3) | Caucasian non-Hispanic |
| 16 (4.1) | Black non-Hispanic |
| 22 (5.6) | Hispanic |
| 4 (1.0) | Other |
| Education | |
| 36 (9.2) | Some high school or less |
| 99 (25.4) | High school graduate |
| 158 (40.5) | Some college or technical school |
| 97 (24.9) | College graduate |
| Income | |
| 105 (27.9) | Less than $15,000 |
| 44 (11.7) | Between $15,001 and $25,000 |
| 64 (17.0) | Between $25,001 and $35,000 |
| 52 (13.8) | Between $35,001 and $50,000 |
| 53 (14.1) | Between $50,001 and $75,000 |
| 59 (15.6) | $75,001 or more |
| Using oxygen for COPD | |
| 136 (35.1) | No |
| 252 (64.9) | Yes |
| M (SD) | Variable |
| 26.9 (5.9) | Body mass index |
| 1.7 (1.1) | Number of comorbid conditions |
| 55.4 (27.5) | Pack-years |
| 6.2 (4.9) | Number of years had COPD |
| 3.4 (1.9) | Number of medications for COPD |
Health Behavior and Symptoms
Current smoking status was assessed via two questions: 1) On average, how many cigarettes do you now smoke a day; and 2) How long ago did you quit smoking? For the first question, a response of zero cigarettes was used to indicate that the respondent was a non-smoker. Any response greater than zero indicated that the respondent was a current smoker. For the second question, if the respondent checked the response “not applicable—I still smoke,” they were considered a current smoker. If the respondent wrote in a response how long ago they quit, they were considered a non-smoker. Respondents had to answer both questions and provide a consistent answer to the two questions to be classified as either a current smoker or a non-smoker.
Urgent health care utilization was assessed via a question about the number of emergency room visits for COPD in the past year and a question about the number of hospitalizations for COPD in the past year. Physical activity was measured by the Physical Activity Scale for the Elderly (PASE). The PASE was designed specifically to measure physical activity in older adults,19 has good psychometric properties,19–21 and has been used in studies of COPD.22
Symptoms of depression and anxiety were measured using the depression and anxiety subscales of the Hospital Anxiety and Depression Scale (HADS). The HADS was designed to assess symptoms of depression and anxiety in medically ill patients23 and has been used in many studies of COPD.24, 25 Breathlessness was measured by the Modified Medical Research Council Scale (MRC), a 5-point grading scale for shortness of breath26 that is predictive of 5-year survival among people with COPD.27 Health-related quality of life (HRQL) was measured by the Total Score on the St. George’s Respiratory Questionnaire (SGRQ), a 50-item scale designed for use in patients with airflow limitation.28 On the HADS, MRC, and SGRQ, higher scores indicate more symptoms and more impairment in quality of life.
Data Analysis
First, the distribution of item responses for each of the IPQ-R cause items was inspected to learn which potential causes were most frequently endorsed by individuals with COPD. Next, IPQ-R cause items were factor analyzed using principal axis factoring and varimax rotation. Finally, correlations of the IPQ-R cause scores with health behaviors and symptoms were calculated. Pearson correlations were used for health behaviors and symptoms that were continuous, and point-biserial correlations were used for health behaviors and symptoms that were dichotomous. Predictive Analytics Software (PASW) version 17.0 was used for all analyses.
Results
Preliminary Analyses
Demographic and clinical characteristics of the sample are presented in Table 1. Forty-six percent of participants were female and the majority of participants (59%) were ≥ 65 years old. On average participants had a 55 pack-year history of smoking and had been diagnosed with COPD approximately 6 years prior to participating in the study. Approximately 65% of the sample reported current treatment with oxygen. Table 2 describes the health behaviors and symptoms of the sample. Only 15% of participants were currently smoking. One-quarter of participants had been to the emergency room for COPD in the past year, and 19% had been admitted to the hospital for their COPD in the past year.
Table 2.
Health Behavior and Symptoms
| Variable | N (%) |
|---|---|
| Smoking status | |
| Not current smoker | 326 (85.3) |
| Current smoker | 56 (14.7) |
| Emergency room visits for COPD | |
| None | 272 (74.3) |
| One or more | 94 (25.7) |
| Hospital admissions for COPD | |
| None | 298 (80.8) |
| One or more | 71 (19.2) |
| Variable | M (SD) |
| Physical activity (PASE) | 102.2 (69.9) |
| Symptoms of depression (HADS) | 5.8 (3.6) |
| Symptoms of anxiety (HADS) | 6.5 (4.2) |
| Breathlessness (MRC) | 2.8 (1.1) |
| Impairment in Health-related quality of life (SGRQ) | 46.2 (18.8) |
Analysis of Causal Attributions
Examination of the item response distributions revealed that, not surprisingly, smoking was the most frequently endorsed cause of COPD. Ninety-three percent of respondents indicated that they agreed or strongly agreed that smoking was a cause of their COPD. Respondents also tended to endorse “my own behavior” more generally as a cause of their COPD. Seventy-two percent of respondents indicated that they agreed or strongly agreed that their own behavior was a cause of their COPD. For all other cause items, less than 50% of respondents indicated that they agreed or strongly agreed that the item was a cause of their COPD, although 48.5% of respondents agreed or strongly agreed that pollution was a cause. See Table 3 for the distribution of responses for all cause items.
Table 3.
Distribution of Item Responses and Rotated Factor Loadings
| Causal Attribution | M (SD) | % Agree or Strongly Agree |
Factor Loading |
|---|---|---|---|
| Stress or worry | 2.5 (1.1) | 22.3 | 0.30 |
| Hereditary—it runs in my family | 2.5 (1.1) | 23.6 | N/A |
| A germ or virus | 2.2 (1.0) | 11.9 | 0.12† |
| Diet or eating habits | 2.2 (0.9) | 11.4 | 0.16† |
| Exercise habits/activity patterns | 2.6 (1.1) | 24.6 | 0.13† |
| Chance or bad luck | 2.1 (0.9) | 8.2 | 0.35 |
| Poor medical care in my past | 2.3 (1.1) | 17.0 | 0.28† |
| Pollution in the environment/workplace | 3.2 (1.2) | 48.5 | N/A |
| My own behavior | 3.7 (1.0) | 71.6 | N/A |
| My mental attitude (e.g., thinking about life negatively) | 2.2 (0.9) | 9.9 | 0.59 |
| Family problems or worries caused my COPD | 2.0 (0.8) | 4.8 | 0.63 |
| Overwork | 2.0 (0.8) | 6.9 | 0.52 |
| My emotional state (e.g., feeling down, lonely, anxious, empty) | 2.2 (0.9) | 11.7 | 0.73 |
| Ageing | 2.7 (1.2) | 32.5 | 0.46 |
| Alcohol | 2.0 (0.9) | 7.1 | 0.55 |
| Smoking | 4.4 (0.9) | 92.6 | N/A |
| Accident or injury | 1.9 (0.9) | 4.5 | 0.52 |
| My personality | 2.0 (0.9) | 5.6 | 0.64 |
| Altered immunity | 2.2 (1.0) | 10.9 | 0.36 |
| Infection or pneumonia | 2.8 (1.3) | 36.5 | 0.07† |
Not retained in final 11-item factor due to low factor loading
Next, a factor analysis with principal axis factoring and varimax rotation was conducted to examine the factor structure using all 20 cause items. Four items were identified that stood alone and were not associated with other items (i.e., smoking, heredity, pollution, and my own behavior). The communality values for these items were: hereditary (.14), pollution (.18), my own behavior (.19), and smoking (.19). The communality values for all other items were above .30. Three of these four items were the three most frequently-endorsed causes in this sample, as described above. Given the low communality values and the gap of greater than .10 between these communality values and those of other items, these four items were considered as stand-alone items.
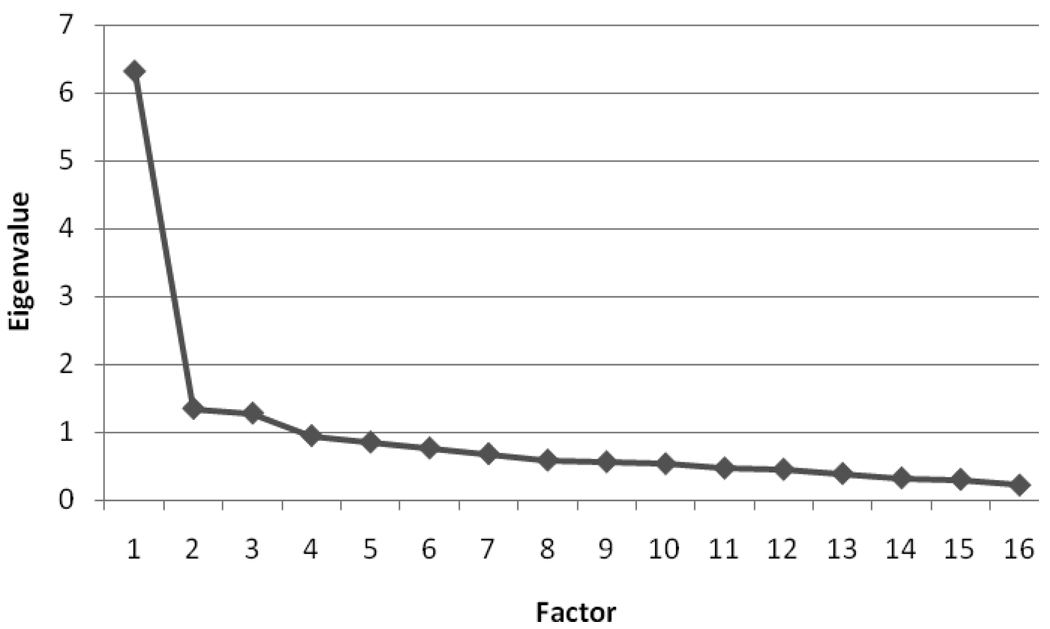
We re-ran the factor analysis with only the 16 remaining items. When only 16 items were used in the factor analysis, all communality values were above .25 and there were no gaps of .10 or more between the communality values of any items. Examination of the eigenvalues suggested a one-factor solution (see Figure 1 for the scree plot). The steep drop in eigenvalues from the first factor (6.31) to the other factors (≤ 1.34) suggests that utilizing a single factor is more appropriate than creating multiple factors. Therefore, we created one large causal attribution factor. The items with the highest rotated factor loadings were emotional state (.73), personality (.64), family problems or worries (.63), and mental attitude (.59). The following five items had rotated factor loadings < .30: diet or eating habits, exercise, poor medical care, infection or pneumonia, and germ or virus. The low factor loadings indicate that these items did not load highly on the factor. These five items were dropped, and the causal attribution factor was created with only the remaining 11 items. Coefficient alpha was high for the 11-item factor (α=.88). See Table 3 for all rotated factor loadings.
Figure 1.
Scree Plot
Association of Causal Attributions with Health Behaviors and Symptoms
The association of causal attributions with the health behaviors and symptoms listed in Table 2 was examined using bivariate correlations. Pearson correlations were calculated between the IPQ-R cause scores and the continuous health behavior variables (i.e., current exercise measured using the PACE, anxiety and depression on the HADS, dyspnea measured by the MRC and impairment in health-related quality of life measured using the SGRQ). Point biserial correlations were calculated between the IPQ-R cause scores and dichotomous health behavior variables (i.e., current smoking, visits to ER, and hospital admissions). To minimize the potential of an inflated Type I error due to multiple comparisons, we conducted a Bonferroni correction resulting in an alpha level of .001 for significance. The causal attribution factor was significantly associated with symptoms of depression (r=.31, p<.001) and anxiety (r=.33, p<.001), and health-related quality of life (r=.28, p<.001). The individual items (i.e., smoking, heredity, pollution, and my own behavior) were not associated with any of the health behaviors or symptoms. See Table 4 for all correlations. In post hoc analyses we also examined the association of two individual items on the IPQ-R (i.e., exercise habits/activity patterns and poor medical care in the past) with selected health behaviors. Attributing COPD to exercise habits/activity patterns was not significantly associated with amount of current physical activity (r=−.004, p>.05). Attributing COPD to poor medical care in the past was not significantly associated with number of emergency room visits for COPD in the past year (r=.08, p>.05) or hospital admissions for COPD in the past year (r=.07, p>.05).
Table 4.
Correlations between IPQ-R Causal Attributions and Health Behaviors
| IPQ-R Causal Attribution | |||||
|---|---|---|---|---|---|
| Health Behavior | 11-Item Causal Attribution Factor |
Heredity Item |
Pollution Item |
My Own Behavior Item |
Smoking Item |
| Current Smoking† | .07 | .05 | .07 | .08 | −.01 |
| ER Visits for COPD† | .05 | .02 | −.01 | .05 | .03 |
| Hospital Admissions for COPD† | .04 | .06 | −.05 | .03 | .01 |
| Physical Activity (PASE Total Score) | −.05 | .15 | .13 | −.03 | .08 |
| Depressive Symptoms (HADS Depression) | .31* | .10 | .12 | .01 | −.02 |
| Anxiety (HADS Anxiety) | .33* | .11 | .11 | −.03 | .01 |
| Breathlessness (MRC) | .12 | .03 | −.08 | −.04 | −.03 |
| Quality of Life (SGRQ Total Score) | .25* | .11 | .13 | −.02 | −.02 |
p<.001
Point biserial correlations were calculated for dichotomous health behavior variables
Discussion
The current study examined COPD patients’ causal attributions for their illness using the IPQ-R, a commonly used measure of causal attributions in chronic illness. We also examined the association between patients’ causal attributions and current health behaviors and emotional adjustment. In our sample of 394 patients with a ≥ 10 pack year smoking history, a factor analysis of the IPQ-R cause items revealed four independent items (i.e., smoking, heredity, pollution, and my own behavior) and one large factor that primarily reflected attributions of psychological causes for COPD (e.g., emotional state, mental attitude, personality). Importantly, the vast majority of patients indicated that smoking was a cause of their COPD and most had quit smoking (i.e., only 15% continued to smoke). These findings suggest that most participants understand that smoking is a primary cause of COPD. It is possible that too few people did not think smoking was a cause of their COPD to detect an association between attributing COPD to smoking and health behaviors and symptoms. In contrast, differences in the degree to which patients endorsed psychological factors as a cause of their COPD (reflected in the large causal attribution factor) was associated with quality of life and current symptoms of depression and anxiety. Patients who attributed the cause of their COPD to psychological factors were more likely to have poorer emotional adjustment and report more significant impact of their COPD on their quality of life. Contrary to our expectations, none of the causal attributions were associated with current smoking, treatment-seeking, or physical activity. As such, causal attributions were not associated with the health-related behaviors assessed in our study.
These findings have implications for understanding the way in which patients with COPD think about the cause of their illness. Our factor analysis revealed several stand-alone items and one large causal attribution factor primarily reflecting psychological causes. This result differs from previous research in other chronic illness populations that has derived a multi-factor structure for the cause items of the IPQ-R. The difference may be due to the importance of smoking, environmental exposure, and heredity in the development of COPD. As the original IPQ authors pointed out, patients with different illnesses may have different primary attributions for the cause of their specific illness (e.g., virus, genetic, or behavior).16 As expected, the majority of our COPD sample endorsed smoking as a cause of their illness, and most were no longer smoking, perhaps reflecting recognition of smoking as the cause of COPD. There was limited variability in how patients responded to the question regarding smoking and their own behavior as a cause, which may account for the lack of association of these items with current health and emotional functioning.
The large factor on the IPQ-R was driven by attributions about psychological functioning as the cause of illness. The items with the highest factor loadings were emotional state, personality, family problems or worries, and mental attitude. Patients who attributed the cause of their COPD to psychological aspects of their history were likely to have greater current symptoms of depression and anxiety, and have poorer quality of life. Thus, attributing COPD to causes other than smoking, and particularly to psychological causes, may be important for current emotional well-being in COPD. It is possible that attributions to psychological concerns in the past may predispose patients to experience more depression and anxiety as their disease progresses. Alternatively, it is possible that emotion or the tendency to experience negative affect is a confounding factor, such that negative affectivity directly affects both a patients’ belief that their past psychological state caused COPD and the tendency to experience more depression and anxiety. Future longitudinal studies that identify patients’ causal attributions for COPD near the time of diagnosis and subsequently follow them over time to determine medical and emotional outcomes will be important in clarifying the direction of the association.
The factor analysis also revealed that five items on the IPQ-R had low factor loadings on the overall causal attribution factor in our sample (i.e., germ or virus, eating habits, exercise habits, poor medical care, and infection or pneumonia). Of note, we had added two of these items (i.e., exercise habits and infection or pneumonia) to the original IPQ-R. The previous study by Scharloo and colleagues18 in COPD derived four factors, but eliminated two factors due to low internal reliability. The two retained factors reflected psychological attributions and behavioral attributions. Notably, four of the five items on Scharloo’s psychological attributions factor were the items that loaded most strongly on our large factor. Scharloo’s behavioral attributions factor included two items —smoking and my own behavior—which were the two most frequently-endorsed items in our sample. The psychological attributions factor in Scharloo’s sample was associated with the mental health subscale of the RAND SF-36 Health Survey and the emotions subscale of the Quality of Life for Respiratory Illness Questionnaire (QoLRIQ). This is similar to the significant correlations between our large factor and symptoms of depression and anxiety. In Scharloo and colleagues’ sample, behavioral attributions were not associated with mental health, emotions, or any aspect of quality of life. This is similar to our observation that endorsing smoking or my own behavior as a cause of COPD was not associated with health behavior and symptoms.
Taken together, these results suggest that future research on causal attributions in COPD should expand upon attributions related to smoking behavior, second hand smoke exposure, and other environmental exposures. For example, it would be informative to add items reflecting whether patients attribute their COPD to beginning smoking at a young age, failing past quit attempts, continuing to smoke after noticing respiratory symptoms, and to family members’ and friends’ smoking. It would also be important to retain items related to psychological attributions, as these items are associated with quality of life and symptoms of depression/anxiety among COPD patients. Researchers using the IPQ-R in the future should be aware of its unique performance in COPD and adjust the measure and their scale calculation accordingly. Future research should also investigate causal attributions among individuals with COPD who do not have a significant smoking history. Given that the primary risk factor for COPD (a personal history of smoking) does not apply to this group of individuals, their beliefs about the causes of their COPD are likely to differ notably from the findings of the current study.
Several limitations are important when considering the conclusions that can be drawn from the current study. First, all data were collected via an anonymous self-report questionnaire and thus, we did not have documented medical utilization or objective measures of COPD severity such as pulmonary function testing. One proxy measure of disease severity is oxygen use. Approximately 65% of the sample reported current oxygen use. This rate is influenced by the study location in Denver, CO where a somewhat higher number of patients require oxygen given the elevation of approximately 1600 meters above sea level. In addition, one of our study sites is a tertiary care center that specializes in lung diseases, which might lead to higher rates of oxygen use than in a general primary care setting. The relatively high rate of oxygen use in this study may limit the generalizability of our results. Another limitation is that the study was cross sectional; as such, it is not possible to determine the direction of the observed associations. For example, the correlation between symptoms of depression/anxiety and psychological attributions may be because patients think that their depression/anxiety caused their COPD. Finally, the response rate was 52%. Individuals who chose to return our questionnaire may not be representative of the entire population of patients being treated for COPD.
Findings from the current study extend the previous literature by examining the causal attributions for illness in a large sample of COPD patients who have at least a ten pack-year history of smoking. We found that on the IPQ-R smoking, heredity, pollution, and my own behavior were independent causes that were endorsed by patients with COPD, and that psychological attributions for COPD can be grouped into a single factor. An important contribution of the study was our examination of response distributions and careful consideration of factor analytic results. Future studies of causal attributions in COPD should more carefully examine smoking and personal behavior, as they appear to be frequently endorsed in COPD but make up only two items on the IPQ-R. The observed associations of psychological attributions with health related quality of life and symptoms of depression and anxiety suggest that patients who attribute their COPD to causes other than smoking may have a higher risk for poor emotional outcomes. Longitudinal studies will be important to tease apart whether psychological attributions contribute to future emotional symptoms or whether patients with more depression and anxiety are also those who tend to consider their own emotional state as a cause of their COPD.
Acknowledgments
We thank Lee Anna Clark, Ph.D. for consultation regarding the statistical analysis. We also thank Richard Albert, MD; Thomas MacKenzie, MD, MSPH; Holly Batal, MD, MBA; Rebecca Hanratty, MD; and Jeanne Rozwadowski, MD; for their help recruiting participants for this study. This study was supported by NIH F32HL083687 awarded to Kristen Holm, Ph.D and supported in part by Colorado CTSA grant 1 UL1 RR025780 from NCRR/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leventhal H, Nerenz D, Steele D. Illness representations and coping with health threats. In: Baum A, Tylor S, Singer J, editors. Handbook of psychology and health. Hillsdale, NJ: Erlbaum; 1984. pp. 219–252. [Google Scholar]
- 2.Leventhal H, Benyamini Y, Brownlee S, et al. Illness representations: Theoretical Foundations. In: Petrie K, Weinman J, editors. Perceptions of health and illness: current research and applications. Amsterdam: Harwood Academic Publishers; 1997. pp. 19–45. [Google Scholar]
- 3.Hermele S, Olivo EL, Namerow P, et al. Illness representations and psychological distress in patients undergoing coronary artery bypass graft surgery. Psychology Health & Medicine. 2007;12:580–591. doi: 10.1080/13548500601162705. [DOI] [PubMed] [Google Scholar]
- 4.Scharloo M, Kaptein AA, Weinman J, et al. Illness perceptions, coping and functioning in patients with rheumatoid arthritis, chronic obstructive pulmonary disease and psoriasis. Journal of Psychosomatic Research. 1998;44:573–585. doi: 10.1016/s0022-3999(97)00254-7. [DOI] [PubMed] [Google Scholar]
- 5.Scharloo M, Kaptein AA, Weinman JA, Hazes JM, Breedveld FC, Rooijmans HG. Predicting functional status in patients with rheumatoid arthritis. Journal of Rheumatology. 1999;26:1686–1693. [PubMed] [Google Scholar]
- 6.Jopson NM, Moss-Morris R. The role of illness severity and illness representations in adjusting to multiple sclerosis. Journal of Psychosomatic Research. 2003;54:503–511. doi: 10.1016/s0022-3999(02)00455-5. discussion 13–4. [DOI] [PubMed] [Google Scholar]
- 7.Petrie K, Moss-Morris R, Weinman J. The impact of catastrophic beliefs on functioning in chronic fatigue syndrome. Journal of Psychosomatic Research. 1995;39:31–37. doi: 10.1016/0022-3999(94)00071-c. [DOI] [PubMed] [Google Scholar]
- 8.Horne R, James D, Petrie K, Weinman J, Vincent R. Patients' interpretation of symptoms as a cause of delay in reaching hospital during acute myocardial infarction. Heart. 2000;83:388–393. doi: 10.1136/heart.83.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry K, Petrie KJ, Ellis CJ, Horne R, Moss-Morris R. Symptom expectations and delay in acute myocardial infarction patients. Heart. 2001;86:91–93. doi: 10.1136/heart.86.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsey SD, Sullivan SD. The burden of illness and economic evaluation for COPD. European Respiratory Journal - Supplement. 2003;41:29s–35s. doi: 10.1183/09031936.03.00078203. [DOI] [PubMed] [Google Scholar]
- 11.Scharloo M, Kaptein AA, Weinman JA, Willems LN, Rooijmans HG. Physical and psychological correlates of functioning in patients with chronic obstructive pulmonary disease. Journal of Asthma. 2000;37:17–29. doi: 10.3109/02770900009055425. [DOI] [PubMed] [Google Scholar]
- 12.Howard C, Hallas CN, Wray J, Carby M. The relationship between illness perceptions and panic in chronic obstructive pulmonary disease. Behav Res Ther. 2009;47:71–76. doi: 10.1016/j.brat.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Kaptein AA, Scharloo M, Fischer MJ, et al. Illness perceptions and COPD: an emerging field for COPD patient management. Journal of Asthma. 2008;45:625–629. doi: 10.1080/02770900802127048. [DOI] [PubMed] [Google Scholar]
- 14.Rennard S. Treatment of stable chronic obstructive pulmonary disease. Lancet. 2004;364:791–802. doi: 10.1016/S0140-6736(04)16941-9. [DOI] [PubMed] [Google Scholar]
- 15.Strassels S. Economic consequences of chronic obstructive pulmonary disease. Current Opinion in Pulmonary Medicine. 1999;5:100–104. doi: 10.1097/00063198-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Weinman J, Petrie KJ, Moss-Morris R, Horne R. The illness perception questionnaire: a new method for assessing the cognitive representation of illness. Psychology and Health. 1996;11:431–445. [Google Scholar]
- 17.Moss-Morris R, Weinman J, Petrie KJ, Cameron LD, Buick D, Horne R. The revised illness perception questionnaire (IPQ-R) 2002 [Google Scholar]
- 18.Scharloo M, Kaptein AA, Schlosser M, et al. Illness perceptions and quality of life in patients with chronic obstructive pulmonary disease. Journal of Asthma. 2007;44:575–581. doi: 10.1080/02770900701537438. [DOI] [PubMed] [Google Scholar]
- 19.Washburn RA, Smith KW, Jetter AM, Janney CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 20.Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Med Sci Sports Exerc. 2001;33:962–970. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Washburn RA, McAuley E, Katula J, Mihalko SE, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 22.Berry MJ, Rejeski WJ, Adair NE, Ettinger WH, Zaccaro DJ, Sevick MA. A randomized, controlled trial comparing long-term and short-term exercise in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2003;23:60–68. doi: 10.1097/00008483-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 24.Hajiro TK, Nishimura M, Tsukino M, Ikeda A, Oga T. Stages of disease severity and factors that affect the health status of patients with chronic obstructive pulmonary disease. Respir Med. 2000;94:841–846. doi: 10.1053/rmed.2000.0804. [DOI] [PubMed] [Google Scholar]
- 25.Okubadejo AA, Jones PW, Wedzicha JA. Quality of life in patients with chronic obstructive pulmonary disease and severe hypoxemia. Thorax. 1996;51:44–47. doi: 10.1136/thx.51.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;5147:257–266. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121:1434–1440. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- 28.Barr JT, Schumacher GE, Freeman S, LeMoine M, Bakst AW, Jones PW. American translation, modification, and validation of the St. George's Respiratory Questionnaire. Clin Ther. 2000;22:1121–1145. doi: 10.1016/S0149-2918(00)80089-2. [DOI] [PubMed] [Google Scholar]