Abstract
Transgenic mice are important tools for our study of breast cancer pathobiology. In order to evaluate changes in cell phenotype with breast cancer progression, we examined vascular and progenitor cell characteristics in tumours derived from MMTV-PyVmT mice. We performed dual-immunofluorescence staining for Tie2, pTie2Y1100, VEGFR2 and PDGFR-β and the pan-endothelial marker PECAM-1 (CD31) in 39 tumours from MMTV-PyVmT transgenic mice grouped by nuclear grade and tumour morphology. Immunohistochemical staining for Aldh1a1 was performed in MMTV-PyVmT-derived tumours and in non-transgenic mouse mammary glands. Tumour blood vessels were heterogeneous in all samples analysed, with the proportion of Tie2-, pTie2 (Y1100)-, VEGFR2- and PDGFR-β-positive tumour blood vessels ranging from 18–98%, 7–40%, 19–86% and 16–94% respectively. We observed a statistically significant difference in vascular pTie2Y1100 levels between low-nuclear-grade tumours and intermediate-/high-nuclear-grade tumours (P = 0.03) and an increase in the proportion of PDGFR-β-positive tumour blood vessels in tumours with high vs. Intermediate-nuclear grade tumours (P < 0.01). Aldh1a1-positive mammary epithelial cells were observed in the terminal end buds of non-transgenic mammary glands and Aldh1a1-positive mammary tumour cells were observed in tumours from MMTV-PyVmT transgenic mice. We observed a decrease in the average number of Aldh1a1-positive cells in tumours with a non-invasive vs. solid morphology (P = 0.03), and in the average number of Aldh1a1-positive mammary tumour cells in low vs. intermediate and low vs. High-nuclear grade tumours (P < 0.001). Our findings suggest heterogeneous expression of several molecules important for tumour angiogenesis and tumour progression that are currently under investigation as therapeutic targets for metastatic breast cancer.
Keywords: Aldh1a1, angiogenesis, endothelial cell, invasive ductal carcinoma, PDGFR-β, Tie2, VEGFR2
It is well established that solid tumour growth beyond a size of 1–2 mm in diameter is angiogenesis dependent, based on the observation that human microscopic, non-angiogenic, tumours can persist in a state of dormancy for long periods of time (Folkman 1971; Black & Welch 1993). Acquisition of an angiogenic tumour phenotype requires an ‘angiogenic switch’, characterized by a shift in the balance of pro- and anti-angiogenic factors in the tumour microenvironment (Folkman et al. 1989; North et al. 2005). In invasive ductal carcinoma of the breast, acquisition of an angiogenic phenotype is thought to preclude transition from ductal hyperplasia to tumour malignancy (Brem et al. 1977). In addition, high microvessel density (MVD) and increased expression of pro-angiogenic factors such as vascular endothelial growth factor (VEGF) have been correlated with high-grade and aggressive ductal carcinoma in situ (DCIS) lesions, high risk of metastatic spread and shorter progression-free and overall survival in node-negative patients (Weidner et al. 1992). Targeting tumour blood vessels using anti-angiogenic agents is an attractive concept in breast cancer therapy. Clinical experience with bevacizumab, a VEGF-neutralizing monoclonal antibody, in metastatic breast cancer has been variable. Contrary to the preclinical efficacy of bevacizumab in treating syngeneic mouse mammary carcinoma models and human breast tumour xenografts (Borgstrom et al. 1999), clinical benefit has been modest and appears to be restricted to specific patient groups (Miller et al. 2005, 2007).
Tie2 (Tunica interna endothelial cell kinase) is an endothelial receptor tyrosine kinase that functions complementary to VEGF-dependent signalling by promoting blood vessel stability and pericyte recruitment. It was initially described as the second member of an orphan receptor tyrosine kinase family, expressed primarily by endothelial cells, important during vascular development (Dumont et al. 1993). Tie2−/− mice are embryonic lethal E9.5-12.5, as the mice fail to develop a normal hierarchy of vascular and lymphatic elements, and exhibit blood vessels with evidence of decreased association between endothelial cells and pericytes (Dumont et al. 1994). Tie2-dependent signalling can promote endothelial differentiation, survival (through increased survivin production), chemotaxis and reduced permeability (Eklund & Olsen 2006); hence, Tie2 is a potentially useful target for anti-angiogenic therapy.
We have previously identified heterogeneity in tumour blood vessel expression of Tie2, relative to the pan-endothelial marker PECAM-1 (CD31), in a number of different tumour types including breast cancer. The degree of heterogeneity was found to be dependent on tumour type and stage of tumour progression, and impacted response to targeted anti-angiogenic therapy directed at Tie2-dependent signalling in models of human melanoma and colorectal carcinoma (Fathers et al. 2005). As heterogeneous expression of Tie2 and other angiogenic receptors seem to be a characteristic feature of each tumour type, cancer specific responses to Tie2 targeted therapy may be expected, yet little is known about the vascular phenotype of mammary cancer vessels in this context. In the present study, we demonstrate heterogeneous expression and activation of Tie2, as well as expression of the endothelial receptor VEGFR2 and the pericyte receptor platelet derived growth factor receptor (PDGFR)-β, during tumour progression in mammary tumours from mice expressing the polyomavirus middle-T antigen under control of the mouse mammary tumour virus promoter (MMTV-PyVmT) (Guy et al. 1992). Furthermore, we demonstrate a statistically significant increase in Tie2 activation and recruitment of tumour pericytes in MMTV-PyVmT mammary tumour blood vessels with increasing nuclear grade. MMTV-PyVmT transgenic mice are a well-established model of human metastatic breast cancer (Guy et al. 1992; Maglione et al. 2001, 2004; Lin et al. 2003; Borowsky et al. 2005); thus, our findings better characterize the availability of relevant target molecules for anti-angiogenic therapies in breast cancer.
The presence of aldehyde dehydrogenase 1a1 (Aldh1a1)-positive mammary tumour cells, which possess stem and/or progenitor cell properties, is associated with hormone receptor negativity, HER-2 overexpression, increased tumour proliferation, poor prognosis and resistance to therapy in human breast cancers (Ginestier et al. 2007; Morimoto et al. 2009; Tanei et al. 2009). In the present study, we demonstrate selective expression of Aldh1a1 by basal epithelial cells, located in terminal end buds of normal murine mammary glands, suggesting the utility of this marker in identifying murine mammary cells with stem/progenitor cell properties. Surprisingly, we report a statistically significant decrease in the number of aldh1a1-positive mammary tumour cells with tumour progression in tumours derived from MMTV-PyVmT mice, independent of tumour vascular phenotype, pericyte recruitment or MVD.
Materials and methods
Mice
All animal studies were performed according to regulations of the Canadian Council on Animal Care as supervised by the local Animal Care Committee at the University of Guelph. Male MMTV-PyVmT mice on an friend leukaemia virus 1b allele mice (FVB) background (obtained from the Mouse Models of Human Cancer Consortium, Frederick, MD) were bred to female FVB mice lacking the MMTV-PyVmT transgene to obtain female hemizygous MMTV-PyVmT mice, which were used for all subsequent analysis. Tumours were removed from female hemizygous mice euthanized at 35, 63 and 77 days of age, as these time points have been previously reported to correspond to stages of human breast cancer progression from premalignant hyperplasia (35 days) to invasive carcinoma (77 days) (Lin et al. 2003). Tissue was divided for formalin-fixation and paraffin embedding, and snap-freezing with liquid N2 in OCT cryomatrix.
Histopathology
Formalin-fixed, paraffin-embedded sections of wildtype mammary glands and MMTV-PyVmT tumours were de-paraffinized and stained with hematoxylin and eosin (H&E; Sigma-Aldrich, Toronto, ON, Canada). Tumour morphology was assessed under 100× magnification with the most advanced structure in each lesion scored in a blinded fashion. Tumours with a well-defined and non-invasive hyperplastic, acinar, glandular, cribiform or papillary morphologies were classified as ‘non-invasive’; acinar, glandular, cribiform or papillary tumours exhibiting invasion into the tissue stroma were classified as ‘focally invasion’; poorly differentiated tumours with widespread invasion were classified as ‘solid’. Nuclear grade was assessed and scored as low, intermediate or high in a blinded fashion under 400× magnification. Briefly, low-grade lesions contained cells with heterochromatic nuclei relatively uniform in size and shape with a slight increase in nuclear/cytoplasmic ratio when compared to normal ductal cells; intermediate grade lesions exhibited an increasing number of cells with euchromatic nuclei, moderate heterogeneity in nuclear size and shape and a moderate increase in nuclear/cytoplasmic ratio; high-grade lesions exhibited nuclear pleomorphism, with a marked increase in nuclear/cytoplasmic ratio and clearly visible nucleoli.
Dual Immunofluorescence for Tie2 and CD31
Cryosections of MMTV-PyVmT tumours were air dried for 30 min, fixed in acetone (Fisher, Nepean, ON) at −20 °C for 10 min and air dried for an additional 15 min. Blocking was performed with 10% normal donkey serum (Sigma-Aldrich) for 1 h. Slides were incubated with a 1:100 dilution of rabbit anti-Tie2 antibody (C-20; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h followed by a 1:300 dilution of Alexa Fluor 488 conjugated donkey anti-rabbit secondary antibody (Invitrogen, Burlington, ON, Canada) for 30 min. A 1:50 dilution of rat anti-CD31 antibody (Hycult Biotechnology, Uden, Netherlands) was incubated with slides overnight at 4 °C. Sections were then incubated with a 1:200 dilution of Cy3-conjugated donkey anti-rat secondary antibody (Jackson Immunoresearch, West Grove, PA, USA) for 30 min. Stained slides were stored on a flat surface at 4 °C in the dark until imaged.
Dual Immunofluorescence for pTie2 (Y1100) and CD31
Cryosections of MMTV-PyVmT tumours were incubated overnight at −20 °C, added directly to acetone at −20 °C for 10 min for fixation, and air dried for 15 min at room temperature. Blocking was performed with a 10% normal donkey serum solution (Sigma-Aldrich) for 1 h followed by incubation with a 1:100 dilution of rabbit anti-pTie2Y1100 antibody (R&D Systems, Minneapolis, MN, USA) for 1 h. Sections were incubated in a 1:300 dilution of Alexa Fluor 488-conjugated donkey anti-rabbit secondary antibody (Invitrogen) for 30 min. Slides were then stained for CD31 as described earlier. Stained slides were stored on a flat surface at 4 °C in the dark until imaged.
Dual Immunofluorescence for VEGFR2 and CD31
Formalin-fixed, paraffin-embedded sections of MMTV-PyVmT tumours were de-paraffinized and subjected to antigen retrieval in a Tris-EDTA buffer solution (pH 9) maintained at 95 °C for 20 min. Sections were incubated in DAKO Protein Block (DakoCytomation, Mississauga, ON, Canada) for 1 h, a 1:100 dilution of goat anti-CD31 (M-20; Santa Cruz Biotechnology) for 1 h and a 1:200 dilution of Alexa Fluor 488-conjugated donkey anti-goat secondary antibody (Invitrogen) for 30 min. Slides were then incubated with a 1:100 dilution of rabbit anti-VEGFR2 antibody (Cell Signaling Technology, Danvers, MA, USA) for 1 h and a 1:200 dilution of Cy3-conjugated goat anti-rabbit secondary antibody (Jackson Immunoresearch) for 30 min. Stained slides were stored on a flat surface at 4 °C in the dark until imaged.
Dual Immunofluorescence for PDGFR-β and CD31
Formalin-fixed, paraffin-embedded sections of MMTV-PyVmT tumours were de-paraffinized and subjected to antigen retrieval in a Tris-EDTA buffer as described earlier. Following blocking in a 10% normal donkey serum solution (Sigma-Aldrich, Mississauga, ON, Canada), slides were incubated with a 1:100 dilution of goat anti-CD31 (M-20; Santa Cruz Biotechnology) for 1 h and a 1:200 dilution of Alexa Fluor 546-conjugated donkey anti-goat secondary antibody (Invitrogen) for 30 min. Slides were then incubated with a 1:100 dilution of rabbit anti-PDGFR-β antibody (Epitomics, Burlingame, CA, USA) and a 1:200 dilution of Alexa Fluor 488-conjugated donkey anti-rabbit secondary antibody (Invitrogen) for 30 min. Stained slides were stored on a flat surface at 4 °C in the dark until imaged.
Blood Vessel Assessment
Expression patterns of Tie2, pTie2 (Y1100), VEGFR2 and PDGFR-β were analysed in a blinded fashion. Five random fields of view of each tumour were captured at a 200× magnification using QCapture software calibrated to a Leica DMLB microscope fitted with a Q imaging QICAM fast1394 digital camera (Leica, Richmond Hill, ON, Canada). Images were captured from each field of view under filters representing the excitation wavelengths of red and green. Red and green images were merged, and the number of marker-positive (Yellow; expressing the protein of interest and CD31), marker-negative (Red; expressing CD31 only) and total tumour blood vessels per field were counted using the merged images. MVD was determined by dividing the total number of blood vessels per field of view, to obtain a value expressed as number of blood vessels per mm2. Blood vessels were considered positive for the marker in question when all or part of the vessel was labelled. This method of assessment thus includes vessels with a ‘composite’ type of expression pattern (Fathers et al. 2005) into the positive vessel category.
Immunohistochemistry for Aldh1a1
Formalin-fixed, paraffin-embedded sections of MMTV-PyVmT tumours were de-paraffinized, subjected to antigen retrieval in a 10 mM sodium citrate buffer (pH 6) for 10 min at 100 °C, and incubated in 3% hydrogen peroxide for 15 min. Blocking was performed with a 10% normal goat serum solution (Sigma-Aldrich) for 1 h. Slides were incubated with a 1:50 dilution of rabbit anti-Aldh1a1 antibody (Epitomics) for 1 h, followed by a 1:200 dilution of biotinylated goat anti-rabbit secondary antibody (Vector Laboratories, Burlington, ON, Canada) for 30 min. Slides were treated with RTU Vectastain Elite, ABC reagent (Vector Laboratories) for 30 min followed by DAB chromogen solution (DakoCytomation) for 5 min. Sections were counterstained with Meyer's hematoxylin (Fisher Scientific, Nepean, ON). The number of Aldh1a1-positive tumour cells per high-power field was determined by analysing 10 fields of view at 400× magnification for each tumour section.
Statistical Analysis
The Anderson-Darling test for normality was used to confirm that data approximated a normal distribution. Comparison of means was performed using one-way anova followed by post-hoc analysis using the Bonferroni method. Least squares regression tested for correlation between vascular and cancer stem cell parameters. Differences were considered significant when P < 0.05 for all analysis. Data are expressed as the mean and standard error of the mean (SEM).
Results
Tumour progression in MMTV-PyVmT tumours as assessed by histological parameters
H&E-stained sections from 39 MMTV-PyVmT mammary tumours were scored for tumour morphology and nuclear grade. Overall, 31% (n = 12) of tumours were classified as having an acinar, glandular, papillary and/or cribiform morphology with no evidence of invasion (Figure 1a–d; Figure 2), 31% (n = 12) of tumours had a similar morphology with focal invasion of the tissue stroma (Figure 1e; Figure 2) and 38% (n = 15) of tumours had a solid and/or invasive phenotype (Figure 1f; Figure 2), with the ratio of solid/invasive: focally invasive and non-invasive lesions increasing from 35 to 77 days at tumour collection. The nuclear grade score per lesion was low in 26% (n = 10) of tumours (Figure 1g–h; Figure 2), intermediate (grade 2) in 36% (n = 14) of tumours (Figure 1i,j; Figure 2) and high (grade 3) in 38% (n = 15) of tumours (Figure 1k–l; Figure 2), with the ratio of high: low nuclear grade increasing with age at tumour collection.
Figure 1.
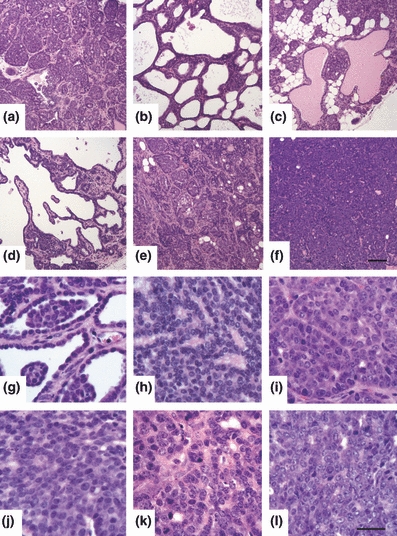
(a–f) Assessment of tumour morphology in H&E-stained MMTV-PyVmT tumours. (a–d) well-defined, non-invasive lesions characteristic of early grade tumours. (a) acinar; tumour comprised of small glandular clusters around small lumens. (b) cribiform; nests of cells forming large lumens. (c) glandular; tumour composed of glands, with some glands dilated by protein-rich secretion. (d) papillary; tumour forms epithelial-lined projections with a fibro-vascular core. (e–f) poorly defined, invasive lesions characteristic of mid – late grade tumours. (e) acinar pattern with focal invasion; boundaries between clusters of cells become unclear. (f) solid; tumour is composed of solid sheets of cells, with no glandular differentiation. Scale bar in (f) represents 100 μm. (g–l) Assessment of nuclear grade in H&E stained MMTV-PyVmT tumours. (g, h) grade 1 (low); heterochromatic nuclei relatively uniform in size and shape with a slight increase in nuclear/cytoplasmic ratio as compared to normal ductal cells. (i, j) grade 2 (intermediate); an increasing number of cells have euchromatic nuclei (nucleoli may or may not be visible). Moderate heterogeneity in nuclear size and shape with a moderate increase in nuclear/cytoplasmic ratio. (k, l) grade 3 (high); nuclear pleomorphism, with a marked increase in nuclear/cytoplasmic ratio and clearly visible nucleoli. Scale bar in (l) represents 30 μm.
Figure 2.
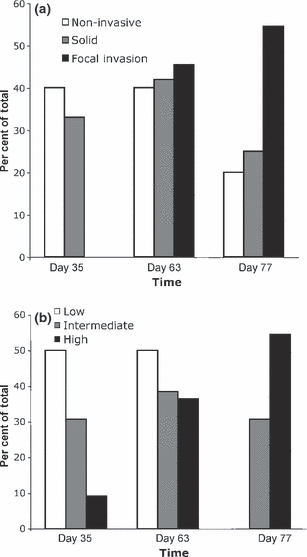
Histological assessment of mammary tumours in 39 lesions from MMTV-PyVmT transgenic mice. H&E-stained sections were used to score tumour morphology (a) and nuclear grade (b). The proportion of tumours with each score is presented from mice euthanized at 35, 63 and 77 days.
Heterogeneity of blood vessels in MMTV-PyVmT mammary tumours
To assess vascular phenotype in mammary carcinoma, serial sections of tumours from MMTV-PyVmT transgenic mice were subjected to dual-immunofluorescence for Tie2, pTie2 (Y1100), VEGFR2 and PDGFR-β, using CD31 as a pan-endothelial marker (Figure 3). Expression of Tie2, pTie2 (Y1100) and VEGFR2 by tumour blood vessels, and PDGFR-β by tumour blood vessel-associated pericytes, was heterogeneous in all samples examined (Figure 3). The average relative proportion of Tie2-, pTie2 (Y1100)-, VEGFR2- and PDGFR-β-positive tumour blood vessels ranged from 18–98%, 7–40%, 19–86% and 16–94%, respectively.
Figure 3.
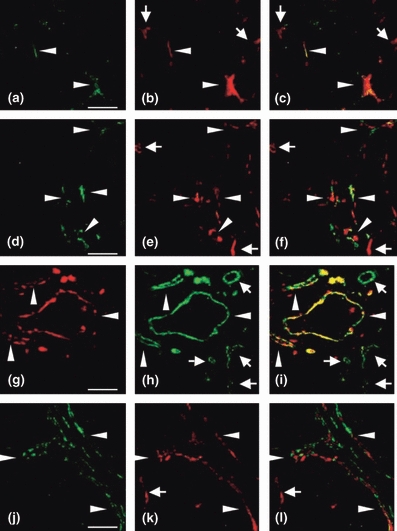
Immunohistochemical evidence for vascular heterogeneity in mammary tumours obtained from MMTV-PyVmT transgenic mice. (a–c) Immunofluorescent staining for Tie2 (a, green) and CD31 (b, red), merged in panel (c), demonstrating Tie2-negative (arrows) and Tie2-positive (arrowheads) tumour blood vessels. (d–f) Dual immunofluorescence for pTie2 (d, green) and CD31 (e, red), merged in panel (f), demonstrating pTie2-negative (arrows) and pTie2-positive (arrowheads) tumour blood vessels. (g–i) Dual immunofluorescence for VEGFR-2 (G, red) and CD31 (h, green), merged in panel (i), demonstrating VEGFR-2-negative (arrows) and VEGFR-2-positive (arrowheads) tumour blood vessels. (j–l) Dual immunofluorescence for PDGFR-β (j, green) and CD31 (k, red), merged in panel (l), demonstrating PDGFR-β-negative (arrows) and PDGFR-β-positive (arrowheads) tumour blood vessels. Scale bars in (a, d, g, j) represent 100 μm.
Tumour vascular phenotype is not dependent on tumour morphology or MVD in MMTV-PyVmT mammary tumours
Tumour vascular phenotype was assessed with tumours classified by morphology, as described earlier. No statistically significant differences were observed in the expression of Tie2, pTie2Y100 or VEGFR2 by tumour blood vessels, or PDGFR-β-positive blood vessel-associated pericytes, between tumours classified as non-invasive, focally invasive or solid (Table 1). In addition, expression of Tie2, pTie2Y1100, VEGFR2 and PDGFR-β were unrelated, and expression of all receptors was not significantly associated with tumour MVD (data not shown).
Table 1.
Expression of Tie2, pTie2 (Y1100) and VEGFR-2 by tumour blood vessels, and PDGFR-β by tumour pericytes, was assessed in relation to tumour morphology in 39, 31, 18 and 32 MMTV-PyVmT tumours, respectively, using dual-immunofluorescence (using CD31 as a pan-endothelial marker). The average relative proportion of Tie2-, pTie2 (Y1100)-, VEGFR-2- and PDGFR-β-positive vessels ranged from 18–98%, 7–40%, 19–86% and 16–94%, respectively. No significant differences were observed in the expression of any vascular or pericyte marker between tumours grouped by tumour morphology (P > 0.05)
| % Tie2+ | % pTie2(Y1100)+ | % PDGFR-β+ | % VEGFR2+ | |
|---|---|---|---|---|
| Non-invasive* | 57.3 ± 9.3 | 19.9 ± 2.9 | 66.3 ± 3.7 | 60.7 ± 4.9 |
| Focal invasion | 62.7 ± 5.7 | 23.0 ± 2.3 | 62.2 ± 6.2 | ND† |
| Solid | 61.8 ± 5.9 | 27.8 ± 2.8 | 72.5 ± 3.8 | 57.3 ± 6.6 |
Represents tumours with morphologies classified as ductal hyperplasia, acinar, cribiform, glandular and papillary with no evidence of invasion into the local tissue stroma.
No tumours classified as ‘focally invasive’ were examined for VEGFR-2 expression.
Increased Tie2 activation and recruitment of tumour pericytes with increasing nuclear grade in MMTV-PyVmT mammary tumour blood vessels
The average proportion of Tie2- or VEGFR2-positive vessels did not differ significantly with MMTV-PyVmT mammary tumours grouped by nuclear grade (Figure 4a,d). Interestingly, we observed a statistically significant difference in vascular pTie2Y1100 levels between low-nuclear-grade tumour and intermediate-/high–nuclear-grade tumours (*P = 0.03), indicating an increased level of Tie2 activation at tyrosine residue Y1100 with increasing tumour aggressiveness (Figure 4b). In addition, we observed a statistically significant increase in the proportion of PDGFR-β-positive tumour blood vessels in tumors with high vs. intermediate nuclear grade, indicating an increased proportion of mature blood vessels in more aggressive tumours (Figure 4c).
Figure 4.
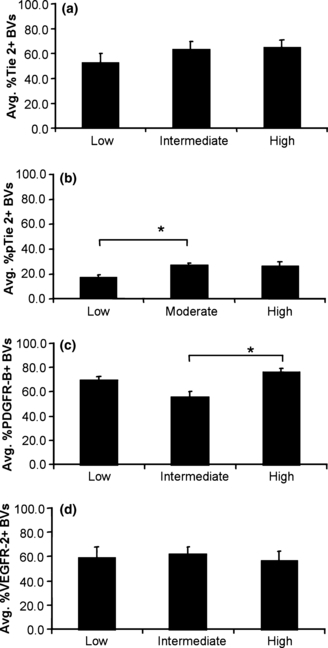
Relative proportion of (a) Tie2-, (b) pTie2 (Y1100)-, (c) PDGFR-β- and (d) VEGFR-2-positive blood vessels in MMTV-PyVmT tumours grouped by nuclear grade (Mean, SEM). No significant differences in vascular expression of (a) Tie2 or (d) VEGFR-2 were observed in tumours assessed as low, intermediate or high nuclear grade (P > 0.05). (b) The proportion of pTie2 (Y1100)-positive blood vessels increased significantly in tumours with intermediate (mean = 26.9%) vs. low (mean = 17.2%) nuclear grade (*P = 0.03). (c) The proportion of PDGFR-β-positive blood vessels increased significantly in tumours with high (mean = 56%) vs. intermediate (mean = 76%) nuclear grade (*P < 0.01).
Aldh1a1 expression in normal mammary gland and MMTV-PyVmT derived tumours during tumour progression
Expression of Aldh1a1 in the normal mouse mammary gland, as assessed by immunohistochemistry, was preferentially found in basal cells of the terminal end buds, indicating that Aldh1a1-positive cells are likely mammary progenitor cells (Figure 5a,b). Expression of Aldh1a1 was further assessed in 23 MMTV-PyVmT tumours (Figure 5c,d); the average number of Aldh1a1-positive mammary tumour cells per high-power field ranged from 0 to 20.2. Interestingly, there was a statistically significant decrease in the average number of Aldh1a1-positive cells in tumours with a non-invasive vs. solid morphology (*P = 0.03), and in the average number of Aldh1a1-positive mammary tumour cells in low vs. intermediate and low vs. High-nuclear-grade tumours (**P < 0.001) (Figure 5e,f). The number of Aldh1a1-positive cells did not correlate with tumour vascular expression of Tie2, pTie2 (Y1100), PDGFR-β or VEGFR2.
Figure 5.
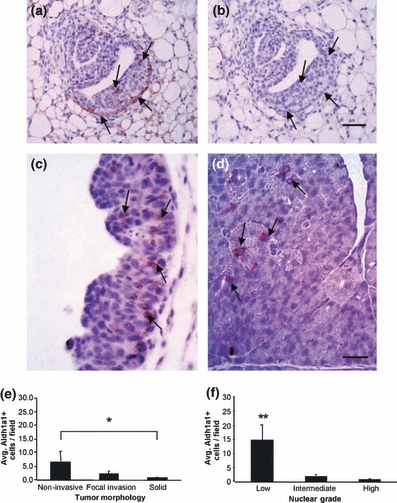
(a–c) Immunohistochemistry identifying Aldh1a1-positive progenitor cells in normal mammary tissue from wt and MMTV-PyVmT mice. (a) Tissue from 5-week-old mouse showing Aldh1a1 positive progenitor cells in mammary gland terminal end buds (brown reaction product with hematoxylin counter stain; arrows). (b) represents immunohistochemistry negative control of adjacent section. Scale bar = 20 μm. (c, d) Arrows indicate Aldh1a1 positive tumour cells (brown) in MMTV-PyVmT tumours; scale bar in c = 100 μm and in d = 25 μm. (e, f) Number of Aldh1a1-positive tumour cells in MMTV-PyVmT tumours grouped by tumour morphology or nuclear grade (Mean, SEM). (e) A significant decrease in the average number of Aldh1a1-positive cells was observed in tumours with a non-invasive vs. solid morphology (*P = 0.03). (f) A significant decrease in the average number of Aldh1a1-positive mammary tumour cells was also observed in low vs. intermediate and low vs. high-nuclear-grade tumours (**P < 0.001).
Discussion
To better understand some of the factors that may be responsible for mixed clinical results of targeted anti-angiogenic therapies in metastatic breast cancer, we investigated the expression and level of activation of the endothelial receptor tyrosine kinase Tie2, which plays a complementary role to VEGF-mediated tumour angiogenic signalling by modulating pericyte recruitment and blood vessel quiescence. Previous work in our laboratory has identified heterogeneous expression of Tie2 by tumour blood vessels. We have previously established that the degree of heterogeneity of Tie2 expression is likely dependent on tumour type and progression in human melanoma and colorectal carcinoma, and impacts tumour response to targeted anti-Tie2 therapy (Fathers et al. 2005). Here, we report heterogeneous expression of Tie2, as well as phosphorylated Tie2 (pTie2Y1100) and VEGFR2 by tumour blood vessels and PDGFR-β by tumour pericytes, in a transgenic mouse model of metastatic breast cancer.
While increased overall Tie2 levels have been demonstrated in human breast tumours (Hayes et al. 2000; Dales et al. 2003, 2004; Sfiligoi et al. 2003; Van der Auwera et al. 2004; Rmali et al. 2007), there is little information on endothelial-specific expression of Tie2 during disease progression. Vascular expression of Tie2 has previously been characterized in syngeneic transplantation tumour models utilizing spontaneous (Lin et al. 1998) and chemically induced (Stratmann et al. 2001) murine mammary cell lines, which lack many important features of human breast cancer angiogenesis and disease progression.
To investigate the expression of Tie2 and pTie2Y1100 during breast cancer progression, we utilized tumours from MMTV-PyVmT transgenic mice, which share a number of important morphological, angiogenic and molecular features with human invasive ductal carcinoma (Guy et al. 1992; Maglione et al. 2001, 2004; Lin et al. 2003; Borowsky et al. 2005). We found the expression of Tie2 was heterogeneous in all MMTV-PyVmT breast tumours examined; however, it was not related to nuclear grade, tumour morphology or MVD. Angiopoietin 2, which acts as both antagonist and agonist for Tie2 signalling, has been reported to be upregulated in human breast cancer and may be associated with increased metastasis in a Tie2 independent fashion (Imanishi et al. 2007). Mutations in oncogenes and tumour suppressor genes, which drive breast tumour progression and invasion, also regulate the expression of Tie2 and other receptors involved in angiogenesis. (Arbiser et al. 1997; Okada et al. 1998). In addition, trastuzumab (which inhibits HER-2-dependent signalling) decreased expression of multiple pro-angiogenic molecules and increased thrombospondin (TSP)-1 expression (Wen et al. 2006).
Vascular Tie2 expression may also be influenced by endothelial cell identity during angiogenesis. A newly forming microvessel is composed of endothelial tip cells that sample the tissue microenvironment and follow chemoattractants and stalk cells, which form endothelial junctions and take on a mature endothelial phenotype (London et al. 2009). Tip cells express membrane-bound delta-like ligand 4 (Dll4), which signals through Notch on stalk cells, inducing a stalk-phenotype, including Tie2 expression and activation (Hellstrom et al. 2007). In contrast, Notch-dependent signalling is inhibited in tip cells, resulting in increased angiogenesis because of an increased proportion of tip cells, which may not utilize Tie2-dependent signalling (Noguera-Troise et al. 2006). Whether Tie2 negative endothelial profiles seen in our analysis represent stalk cells remains to be investigated.
Functional alterations in mammary gland vasculature can occur prior to observable tumour morphological changes (Lichtenbeld et al. 1998). Orthotopic implantation of human breast tumour-adjacent fragments into mammary fat pads of immune-deficient mice induced angiogenesis more frequently than normal tissue from breast-reduction surgery (Lichtenbeld et al. 1998). Heterogeneity in vascular patterning was reported in 75 patients with similar DCIS lesions, which were characterized by a significant increase in stromal vascularity relative to normal breast, or by a dense rim of microvessels adjacent to the basement membrane (Engels et al. 1997). The distribution of these two distinct vascular patterns varied significantly between patients, as well as within different regions of the same tumour (Engels et al. 1997). In agreement with our data, these results suggest that expression of endothelial receptors in angiogenesis is regulated independent of tumour morphology or MVD.
The distribution of vessels expressing Tie2, phosphorylated at Y1100 (pTie2Y1100) was heterogeneous in MMT-PyVmT tumours and significantly lower relative to native Tie2, indicating Y1100-dependent activation was only occurring in a percentage of Tie2-positive blood vessels. Transgenic mice expressing a mutant form of Tie2 which cannot be phosphorylated at Y1100 (Tie2F1100 mice) die in utero, sharing similar features to Tie2-null embryos including defects in the heart and angiogenesis (Tachibana et al. 2005). Interestingly, perivascular cell recruitment is unaffected in vessels in the head region of Tie2F1100 embryos, suggesting alternate tyrosine residues such as Y1106 and Y1111 may be primarily responsible for vascular stability in these regions (Tachibana et al. 2005). In vitro, co-expression of Tie1 in endothelial cells has been shown to mediate Ang1- and Ang2-dependent Tie2 phosphorylation, without affecting Tie2 receptor expression at the cell membrane (Marron et al. 2007). In vitro and in vivo, Ang2 was found to promote Tie2-dependent pro-angiogenic behaviour in human cord blood-derived endothelial precursor cells (EPCs), but not HUVECs. Further analysis revealed Tie2 receptors on HUVECs were bound by Tie1, while EPC Tie2 receptors were unbound (Kim et al. 2006). Tie1 mRNA expression is increased in breast tumours, and while Tie1 expression and activation have been reported in MCF-7 and inflammatory human breast cancer cell lines in vitro (Rees et al. 2007) (Shirakawa et al. 2002), endothelial Tie1 expression has yet to be characterized in breast tumours. Tie2 and VEGFR2-mediated signalling play complementary roles during tumour angiogenesis. At sites of active remodelling, concurrent expression of the Tie2-ligand Ang2 and VEGF promotes pericyte detachment, endothelial survival and VEGF-induced mitogenesis and migration. In contrast, Ang2-mediated destabilization leads to vessel regression without concurrent expression of VEGF (Holash et al. 1999; Zhang et al. 2003). MMTV-PyVmT mice with conditional endothelial cell overexpression of VEGF have an exuberant angiogenic response that promotes accelerated growth, progression and metastasis (Schoeffner et al. 2005). We also observed heterogeneous expression of VEGFR2 by tumour blood vessels in all MMTV-PyVmT tumours examined. While similar expression patterns have not yet been reported for human breast cancer, our results suggest that specific patient groups may not respond equally to anti-VEGF targeted anti-angiogenic therapies (such as bevacizumab) currently used to treat metastatic breast cancer. Interestingly, differential VEGFR2 expression was not associated with nuclear grade, tumour morphology, MVD or Tie2 expression, indicating factors independent of tumour progression and concurrent Tie2 signalling likely regulate its expression. In contrast to Tie2, VEGFR2 is likely upregulated in tip cells during angiogenesis, where VEGF-VEGFR2-mediated signalling induces the formation of actin-rich filopodia (Jakobsson et al. 2009). Dll4/Notch1 signalling leads to down-regulation of VEGFR2 and up-regulation of VEGFR1 on stalk cells (Jakobsson et al. 2009).
Complementary signalling between Ang1-Tie2 and PDGFBB-PDGFR-β occurs between endothelial cells and pericytes during tumour angiogenesis (Bergers & Song 2005). As a result, PDGFR-β is currently one of the main target molecules utilized in multi-targeted anti-angiogenic therapies (such as sunitinib, a PDGFR, VEGFR and KIT small molecule inhibitor) (Marty & Pivot 2008). We found heterogeneous expression of PDGFR-β by blood vessel–associated pericytes in MMTV-PyVmT tumours, with the proportion of PDGFR-β-positive blood vessels increasing with increasing nuclear grade. However, expression of specific pericyte markers, including desmin, α-smooth muscle actin, PDGFR-β, NG2, RGS-5 and Tem1 is developmental-stage, tumour type and even blood vessel type specific; consequently, it has been a challenge defining pericyte coverage in tumour blood vessels owing to the lack of reliable pan-pericyte markers (Bergers & Song 2005; Christian et al. 2008). Mammary cancer xenografts overxpressing Ang2 had reduced pericyte coverage, reflecting the importance of Tie2 signalling in mural cell recruitment (Reiss et al. 2009). Human breast cancers exhibit a range of pericyte coverage of between 40 and 85% of tumour vessels (Eberhard et al. 2000), similar to the values we report here. Our results highlight the heterogeneous nature of tumour pericytes in MMTV-PyVmT mammary tumours and indicate the potential for increased pericyte-coverage in tumour blood vessels in advancing tumours. Differences in pericyte coverage could have implications for the response of human metastatic breast cancer patients to multi-targeted anti-angiogenic therapies such as sunitinib (Marty & Pivot 2008).
A high level of Aldh1a1 activity is a common feature of stem/progenitor cells in the colon (Huang et al. 2009), hematopoietic system and central nervous system (Levi et al. 2009). In the mammary gland, high Aldh1a1 activity selects for human mammary stem/progenitor cells with the broadest differentiation potential and growth capacity (Ginestier et al. 2007). Increased expression of Aldh1a1 in cancer stem/progenitor cells has been demonstrated in colon (Carpentino et al. 2009) and lung cancer (Patel et al. 2008; Ucar et al. 2009). In invasive ductal carcinoma of the breast, high activity of Aldh1a1 identifies a highly-tumourigenic cell fraction which has self-renewal capacity and ability to form heterogeneous tumours (Ginestier et al. 2007). Alterations in the frequency and distribution of cancer stem/progenitor cells in breast tumours classified according to subtype and tumour progression have implications for therapeutic strategies aimed at the selective targeting of cancer stem/progenitor cells. We found that Aldh1a1-positive cells in normal mammary gland of FVB mice were located in the basal layer of terminal end buds, suggesting that Aldh1a1 is in fact a marker for mammary progenitor cells. Expression of Aldh1a1 in human breast tumours, as assessed by IHC, is associated with high histological and nuclear grade, high mitotic count, hormone receptor-negativity (Morimoto et al. 2009; Nalwoga et al. 2010) epidermal growth factor receptor (EGFR) and HER-2 overexpression (Nalwoga et al. 2010; Morimoto et al. 2009, Park et al. 2010), ki-67 and p53 expression (Morimoto et al. 2009), and a basal-like phenotype (Nalwoga et al. 2010; Park et al. 2010). High Aldh1a1 levels in human breast cancer have also been associated with poor prognosis and resistance to therapy, although not all studies report this (Ginestier et al. 2007; Morimoto et al. 2009; Resetkova et al. 2009; Tanei et al. 2009; Neumeister et al. 2010).
Invasion and metastasis in inflammatory breast cancer are mediated by a cellular component enriched for high Aldh1a1 activity, while expression of Aldh1a1 in patient samples is an independent predictive factor for early metastasis and decreased survival (Charafe-Jauffret et al. 2010). In contrast to data from human breast cancer, the presence of mammary-derived stem/progenitor cells has previously been refuted in MMTV-PyVmT mice using the epithelial progenitor cell markers Sca-1 and keratin 6 (Li et al. 2003). We have demonstrated Aldh1a1-positive cells in early, non-invasive lesions, with a significant drop in the frequency of Aldh1a1-positive cells during tumour progression. Interestingly, MMTV-PymT tumours are considered to have a gene expression profile signature similar to human cancers with a ‘luminal’ phenotype (Herschkowitz et al. 2007), and Aldh1 levels are much lower in human ‘luminal’ breast tumours than in cancers with a basal-like phenotype (Park et al. 2010).
Our results thus confirm the luminal phenotype of mammary cancers in this model and suggest that breast tumour stem/progenitor cells expressing Sca-1, keratin 6 or Aldh1a1 in MMTV-PyVmT tumours may represent different cell populations. In contrast to human metastatic breast cancer, we observed a drop-off in the number of Aldh1a1-positive cells with tumour progression, similar to what has been reported in human ovarian cancer (Chang et al. 2009). This may indicate that cancer cell heterogeneity in MMTV-PyVmT tumours results from clonal evolution of more differentiated cells, rather than from cancer stem/progenitor cells per se, a finding that should be considered when this transgenic model is employed. Taken together our results highlight the need to better characterize the expression of molecules targeted in metastatic breast cancer, with the ultimate goal of selecting patient groups that may respond better to targeted anti-angiogenic and anti-tumour-progenitor cell therapies.
Acknowledgments
We thank the other members of the Coomber laboratory for helpful suggestions and technical support. Special thanks to Alyssa Foulkes, Kirsten Anderson and Mary Fowler (Central Animal Facility, University of Guelph) and Tony Cengija and Barb Mitchell (OVC Isolation Unit) for their assistance with the mice. Dr. Geoff Wood provided invaluable advice regarding tumour histopathology, and William Sears provided support for statistical analysis. This work was supported by an Operating Grant (MOP#81213) to BLC from the Canadian Institutes for Health Research.
Competing interests
The authors declare no conflict of interest.
References
- Arbiser JL, Moses MA, Fernandez CA, et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc. Natl. Acad. Sci. USA. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N. Engl. J. Med. 1993;328:1237–1243. doi: 10.1056/NEJM199304293281706. [DOI] [PubMed] [Google Scholar]
- Borgstrom P, Gold DP, Hillan KJ, Ferrara N. Importance of VEGF for breast cancer angiogenesis in vivo: implications from intravital microscopy of combination treatments with an anti-VEGF neutralizing monoclonal antibody and doxorubicin. Anticancer Res. 1999;19:4203–4214. [PubMed] [Google Scholar]
- Borowsky AD, Namba R, Young LJ, et al. Syngeneic mouse mammary carcinoma cell lines: two closely related cell lines with divergent metastatic behavior. Clin. Exp. Metastasis. 2005;22:47–59. doi: 10.1007/s10585-005-2908-5. [DOI] [PubMed] [Google Scholar]
- Brem SS, Gullino PM, Medina D. Angiogenesis: a marker for neoplastic transformation of mammary papillary hyperplasia. Science. 1977;195:880–882. doi: 10.1126/science.402692. [DOI] [PubMed] [Google Scholar]
- Carpentino JE, Hynes MJ, Appelman HD, et al. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69:8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Liu G, Xue F, et al. ALDH1 expression correlates with favorable prognosis in ovarian cancers. Mod. Pathol. 2009;22:817–823. doi: 10.1038/modpathol.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charafe-Jauffret E, Ginestier C, Iovino F, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin. Cancer Res. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian S, Winkler R, Helfrich I, et al. Endosialin (Tem1) is a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. Am. J. Pathol. 2008;172:486–494. doi: 10.2353/ajpath.2008.070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales JP, Garcia S, Bonnier P, et al. Tie2/Tek expression in breast carcinoma: correlations of immunohistochemical assays and long-term follow-up in a series of 909 patients. Int. J. Oncol. 2003;22:391–397. [PubMed] [Google Scholar]
- Dales JP, Garcia S, Carpentier S, et al. Prediction of metastasis risk (11 year follow-up) using VEGF-R1, VEGF-R2, Tie-2/Tek and CD105 expression in breast cancer (n = 905) Br. J. Cancer. 2004;90:1216–1221. doi: 10.1038/sj.bjc.6601452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont DJ, Gradwohl GJ, Fong GH, Auerbach R, Breitman ML. The endothelial-specific receptor tyrosine kinase, tek, is a member of a new subfamily of receptors. Oncogene. 1993;8:1293–1301. [PubMed] [Google Scholar]
- Dumont DJ, Gradwohl G, Fong GH, et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000;60:1388–1393. [PubMed] [Google Scholar]
- Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp. Cell Res. 2006;312:630–641. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Engels K, Fox SB, Whitehouse RM, Gatter KC, Harris AL. Distinct angiogenic patterns are associated with high-grade in situ ductal carcinomas of the breast. J. Pathol. 1997;181:207–212. doi: 10.1002/(SICI)1096-9896(199702)181:2<207::AID-PATH758>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Fathers KE, Stone CM, Minhas K, et al. Heterogeneity of Tie2 expression in tumor microcirculation: influence of cancer type, implantation site, and response to therapy. Am. J. Pathol. 2005;167:1753–1762. doi: 10.1016/S0002-9440(10)61256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol. Cell. Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AJ, Huang WQ, Yu J, et al. Expression and function of angiopoietin-1 in breast cancer. Br. J. Cancer. 2000;83:1154–1160. doi: 10.1054/bjoc.2000.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M, Phng LK, Hofmann JJ, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- Herschkowitz JI, Simin K, Weigman VJ, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18:5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- Huang EH, Hynes MJ, Zhang T, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi Y, Hu B, Jarzynka MJ, et al. Angiopoietin-2 stimulates breast cancer metastasis through the alpha(5)beta(1) integrin-mediated pathway. Cancer Res. 2007;67:4254–4263. doi: 10.1158/0008-5472.CAN-06-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L, Bentley K, Gerhardt H. VEGFRs and Notch: a dynamic collaboration in vascular patterning. Biochem. Soc. Trans. 2009;37:1233–1236. doi: 10.1042/BST0371233. [DOI] [PubMed] [Google Scholar]
- Kim KL, Shin IS, Kim JM, et al. Interaction between Tie receptors modulates angiogenic activity of angiopoietin2 in endothelial progenitor cells. Cardiovasc. Res. 2006;72:394–402. doi: 10.1016/j.cardiores.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Levi BP, Yilmaz OH, Duester G, Morrison SJ. Aldehyde dehydrogenase 1a1 is dispensable for stem cell function in the mouse hematopoietic and nervous systems. Blood. 2009;113:1670–1680. doi: 10.1182/blood-2008-05-156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Welm B, Podsypanina K, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc. Natl. Acad. Sci. USA. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenbeld HC, Barendsz-Janson AF, van Essen H, Struijker Boudier H, Griffioen AW, Hillen HF. Angiogenic potential of malignant and non-malignant human breast tissues in an in vivo angiogenesis model. Int. J. Cancer. 1998;77:455–459. doi: 10.1002/(sici)1097-0215(19980729)77:3<455::aid-ijc23>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Lin P, Buxton JA, Acheson A, et al. Antiangiogenic gene therapy targeting the endothelium-specific receptor tyrosine kinase Tie2. Proc. Natl. Acad. Sci. USA. 1998;95:8829–8834. doi: 10.1073/pnas.95.15.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Jones JG, Li P, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London NR, Whitehead KJ, Li DY. Endogenous endothelial cell signaling systems maintain vascular stability. Angiogenesis. 2009;12:149–158. doi: 10.1007/s10456-009-9130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione JE, Moghanaki D, Young LJ, et al. Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res. 2001;61:8298–8305. [PubMed] [Google Scholar]
- Maglione JE, McGoldrick ET, Young LJ, et al. Polyomavirus middle T-induced mammary intraepithelial neoplasia outgrowths: single origin, divergent evolution, and multiple outcomes. Mol. Cancer Ther. 2004;3:941–953. [PubMed] [Google Scholar]
- Marron MB, Singh H, Tahir TA, et al. Regulated proteolytic processing of Tie1 modulates ligand responsiveness of the receptor-tyrosine kinase Tie2. J. Biol. Chem. 2007;282:30509–30517. doi: 10.1074/jbc.M702535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty M, Pivot X. The potential of anti-vascular endothelial growth factor therapy in metastatic breast cancer: clinical experience with anti-angiogenic agents, focusing on bevacizumab. Eur. J. Cancer. 2008;44:912–920. doi: 10.1016/j.ejca.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J. Clin. Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Kim SJ, Tanei T, et al. Stem cell marker aldehyde dehydrogenase 1-positive breast cancers are characterized by negative estrogen receptor, positive human epidermal growth factor receptor type 2, and high Ki67 expression. Cancer Sci. 2009;100:1062–1068. doi: 10.1111/j.1349-7006.2009.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalwoga H, Arnes JB, Wabinga H, Akslen LA. Expression of aldehyde dehydrogenase 1 (ALDH1) is associated with basal-like markers and features of aggressive tumours in African breast cancer. Br. J. Cancer. 2010;102:369–375. doi: 10.1038/sj.bjc.6605488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister V, Agarwal S, Bordeaux J, Camp RL, Rimm DL. In situ identification of putative cancer stem cells by multiplexing ALDH1, CD44, and cytokeratin identifies breast cancer patients with poor prognosis. Am. J. Pathol. 2010;176:2131–2138. doi: 10.2353/ajpath.2010.090712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguera-Troise I, Daly C, Papadopoulos NJ, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- North S, Moenner M, Bikfalvi A. Recent developments in the regulation of the angiogenic switch by cellular stress factors in tumors. Cancer Lett. 2005;218:1–14. doi: 10.1016/j.canlet.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Okada F, Rak JW, Croix BS, et al. Impact of oncogenes in tumor angiogenesis: mutant K-ras up-regulation of vascular endothelial growth factor/vascular permeability factor is necessary, but not sufficient for tumorigenicity of human colorectal carcinoma cells. Proc. Natl. Acad. Sci. USA. 1998;95:3609–3614. doi: 10.1073/pnas.95.7.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Lee HE, Li H, Shipitsin M, Gelman R, Polyak K. Heterogeneity for stem cell-related markers according to tumor subtype and histologic stage in breast cancer. Clin. Cancer Res. 2010;16:876–887. doi: 10.1158/1078-0432.CCR-09-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Lu L, Zander DS, Sreerama L, Coco D, Moreb JS. ALDH1A1 and ALDH3A1 expression in lung cancers: correlation with histologic type and potential precursors. Lung Cancer. 2008;59:340–349. doi: 10.1016/j.lungcan.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Rees KA, Singh H, Brindle NP. The receptor tyrosine kinase Tie1 is expressed and activated in epithelial tumour cell lines. Int. J. Oncol. 2007;31:893–897. [PubMed] [Google Scholar]
- Reiss Y, Knedla A, Tal AO, et al. Switching of vascular phenotypes within a murine breast cancer model induced by angiopoietin-2. J. Pathol. 2009;217:571–580. doi: 10.1002/path.2484. [DOI] [PubMed] [Google Scholar]
- Resetkova E, Reis-Filho JS, Jain RK, et al. Prognostic impact of ALDH1 in breast cancer: a story of stem cells and tumor microenvironment. Breast Cancer Res. Treat. 2009;123:97–108. doi: 10.1007/s10549-009-0619-3. [DOI] [PubMed] [Google Scholar]
- Rmali KA, Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. Angiopoietins lack of prognostic significance in ductal mammary carcinoma. Int. Semin. Surg. Oncol. 2007;4:6. doi: 10.1186/1477-7800-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeffner DJ, Matheny SL, Akahane T, et al. VEGF contributes to mammary tumor growth in transgenic mice through paracrine and autocrine mechanisms. Lab. Invest. 2005;85:608–623. doi: 10.1038/labinvest.3700258. [DOI] [PubMed] [Google Scholar]
- Sfiligoi C, de Luca A, Cascone I, et al. Angiopoietin-2 expression in breast cancer correlates with lymph node invasion and short survival. Int. J. Cancer. 2003;103:466–474. doi: 10.1002/ijc.10851. [DOI] [PubMed] [Google Scholar]
- Shirakawa K, Wakasugi H, Heike Y, et al. Vasculogenic mimicry and pseudo-comedo formation in breast cancer. Int. J. Cancer. 2002;99:821–828. doi: 10.1002/ijc.10423. [DOI] [PubMed] [Google Scholar]
- Stratmann A, Acker T, Burger AM, Amann K, Risau W, Plate KH. Differential inhibition of tumor angiogenesis by tie2 and vascular endothelial growth factor receptor-2 dominant-negative receptor mutants. Int. J. Cancer. 2001;91:273–282. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1054>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Jones N, Dumont DJ, Puri MC, Bernstein A. Selective role of a distinct tyrosine residue on Tie2 in heart development and early hematopoiesis. Mol. Cell. Biol. 2005;25:4693–4702. doi: 10.1128/MCB.25.11.4693-4702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanei T, Morimoto K, Shimazu K, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin. Cancer Res. 2009;15:4234–4241. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- Ucar D, Cogle CR, Zucali JR, et al. Aldehyde dehydrogenase activity as a functional marker for lung cancer. Chem. Biol. Interact. 2009;178:48–55. doi: 10.1016/j.cbi.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera I, Van Laere SJ, Van den Eynden GG, et al. Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin. Cancer Res. 2004;10:7965–7971. doi: 10.1158/1078-0432.CCR-04-0063. [DOI] [PubMed] [Google Scholar]
- Weidner N, Folkman J, Pozza F, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J. Natl. Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- Wen XF, Yang G, Mao W, et al. HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006;25:6986–6996. doi: 10.1038/sj.onc.1209685. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yang N, Park JW, et al. Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res. 2003;63:3403–3412. [PubMed] [Google Scholar]


