Table II.
Kinetic Constants
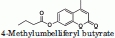 | |||
|---|---|---|---|
| Construct | KM (μM) | kcat (min−1) | kcat/KM (μM−1 min−1) |
| wild-type 1-303 | 162 | 68 | 0.42 |
| Lid-domain mutants | |||
| 1-303 Leu169Ser, Leu176Ser | 136 | 48 | 0.35 |
| 9-393 Leu169Ser, Leu176Ser | 88 | 27 | 0.31 |
| 9-297 Leu169Ser, Leu176Ser | 126 | 26 | 0.21 |
| 1-303 Leu171Gln | 105 | 51 | 0.48 |
| 1-303 Leu167Gln, Leu171Gln | 84 | 59 | 0.71 |
| 1-303 Leu167Gln, Leu174Gln | 84 | 70 | 0.83 |
| 1-303 Leu171Gln, Leu174Gln | 89 | 47 | 0.52 |
| Lid-domain + Surface mutants | |||
| 1-303 Leu169Ser, Leu176Ser, Lys36Ala | 124 | 51 | 0.41 |
| 1-303 Leu169Ser, Leu176Ser, Lys160Ala | 90 | 30 | 0.33 |
| 1-303 Leu169Ser, Leu176Ser, Lys165Ala | 137 | 27 | 0.2 |
| 1-303 Leu169Ser, Leu176Ser, Lys226Ala | 110 | 38 | 0.35 |
| 1-303 Leu169Ser, Leu176Ser, Lys36Ala, Lys226Ala | 123 | 30 | 0.25 |
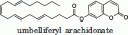 | |||
| wild-type 1-303 | * | * | 0.09 |
| mutant 1-303, Leu169Ser, Leu176Ser | * | * | 0.1 |
Kinetic constants of the various MGL constructs using 4-methylumbelliferyl butyrate or umbelliferyl arachidonate as substrates. Values for the 4-methylumbelliferyl butyrate substrate are the average of 2 or 4 separate assays. kcat/KM values for the umbelliferyl arachidonate substrate are the average values for the hydrolysis of five different substrate concentrations at [S] < KM.
(*)The solubility limit of the umbelliferyl arachidonate substrate did not allow for the determination of KM and kcat.
