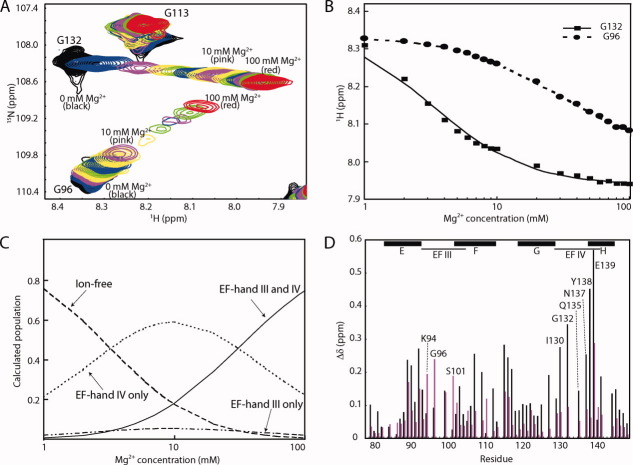
Figure 4.
Mg2+ binding to the CaMC DD mutant. (A) Titration with Mg2+. Superimposition of the 1H-15N HSQC spectra of the CaMC DD mutant with Mg2+ concentrations ranging from 0 to 100 mM. Each spectrum was acquired at 50°C. (B) Titration curve for Mg2+ binding to the CaMC DD mutant. Amide proton chemical shifts assigned to G96 (EF-hand III, circles) and G132 (EF-hand IV, squares), plotted as a function of the Mg2+ concentration. (C) Calculated populations of the different states of the protein, as a function of the Mg2+ concentration. The populations were calculated for KIII = 29.4 mM, KIV = 2.67 mM, and 1 mM protein. (D) Backbone amide chemical shift changes induced by Mg2+ binding. Black bars represent the chemical shift changes between 0 and 10 mM Mg2+, and magenta bars indicate the chemical shift changes between 10 and 100 mM Mg2+. The values were calculated with the following equation: 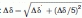 . The signals of E83, I85, N111, E114, V142, and Q143 were overlapped. The chemical shift changes of the D95, N97, G98, I100, V108, R126, A128, D131, D133, G134, and V136 residues were too broad, and are not included in the plot. The binding sequences of the EF-hands are indicated with a line. The helices are indicated by boxes with their names.
. The signals of E83, I85, N111, E114, V142, and Q143 were overlapped. The chemical shift changes of the D95, N97, G98, I100, V108, R126, A128, D131, D133, G134, and V136 residues were too broad, and are not included in the plot. The binding sequences of the EF-hands are indicated with a line. The helices are indicated by boxes with their names.