Figure 7.
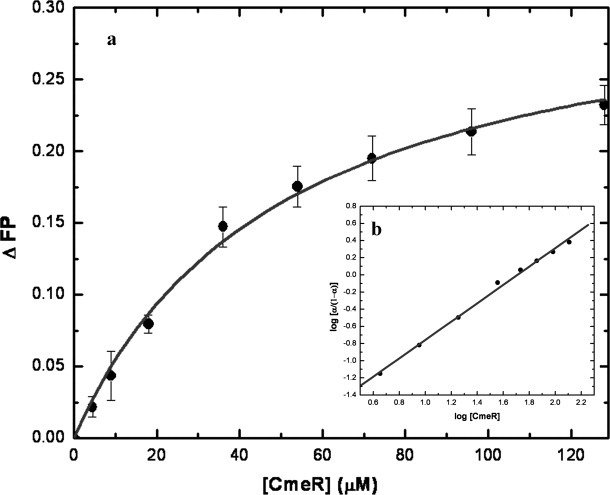
Fluorescence polarization of CmeR with cholyl-lysyl-fluorescein. (a) Binding isotherm of CmeR with cholyl-lysyl-fluorescein, showing a KD of 50.2 ± 0.4 μM, in buffer containing 10 mM Na-phosphate (pH 7.2) and 100 mM NaCl. (b) Hill plot of the data obtained for cholyl-lysyl-fluorescein binding to CmeR. α corresponds to the fraction of bound cholyl-lysyl-fluorescein. The plot gives a slope of 1.07 ± 0.03, indicating a simple binding process with no cooperativity. The interception of the plot provides a KD of 51.1 ± 7.7 μM for the cholyl-lysyl-fluorescein binding.
