Abstract
Animals can acquire behaviours from others, including heterospecifics, but should be discriminating in when and whom to copy. Successful individuals should be preferred as tutors, while adopting traits of poorly performing individuals should be actively avoided. Thus far it is unknown if such adaptive strategies are involved when individuals copy other species. Furthermore, rejection of traits based on tutor characteristics (negative bias) has not been shown in any non-human animal. Here we test whether a choice between two new, neutral behavioural alternatives—breeding-sites with alternative geometric symbols—is affected by observing the choice and fitness of a heterospecific tutor. A field experiment replicated in four different areas shows that the proportion of pied flycatcher females matching the choice of the tit tutor consistently increased with increasing number of offspring in the tit nest, to the extent of nearly complete prevalence in one of the areas when tit fitness was highest. Notably, all four replicates demonstrate rejection of the behaviour of lowest-fitness tutors. The results demonstrate both acquisition and avoidance of heterospecific behavioural traits, based on the perceived (lack of) tutor fitness. This has potential implications for understanding the origin, diversity and local adaptations of behavioural traits, and niche overlap/partitioning and species co-occurrence.
Keywords: copying, learning bias, social learning, niche overlap, animal culture, animal traditions
1. Introduction
Many studies demonstrate potential for non-genetic behavioural trait transmission via copying between animal species [1–5]. While the evolutionary and behavioural ecology of copying has been extensively studied in a conspecific context [6–10], there is little research on whether the predicted processes and strategies apply to interspecific copying. In particular, copying among conspecifics is expected to be adaptive only when it is discriminative: only some individuals or actions are worth copying. Attributes used to select individuals or actions worth copying could be, for example, social prestige, success, size, age or frequency of occurrence [11–15].
The quality of the individual being observed is expected to be one of the most important criteria for discriminative copying [11,12,14]. Individuals measuring high on observable fitness correlates—such as size, age, status or number of offspring—plausibly have better behaviours in their repertoire and make better decisions than individuals measuring low on observable fitness, and should thus be copied more often [12,14]. But because variation in fitness correlates is rarely commensurate in different species, it is unknown whether animals are able to discriminate between observed heterospecific individuals.
A rarely noted complementary of the ‘copy-the-successful’-strategy is that behaviours of manifestly poor individuals should be actively rejected, not merely ignored. While positive bias is evident in many studies (e.g. [16,17]), we are not aware of studies to date discussing or showing active negative bias. This subtle complementary strategy could be important. Copying carries the potential disadvantage of acquiring harmful behaviours [13,14]. Actively avoiding any and all new behaviours observed to be exhibited by poor individuals encountered may focus the observer to a smaller set of better alternatives, or perhaps even allow dropping previously acquired disadvantageous behaviours before they become costly. This ‘pruning’ effect could confer benefits from observing others, even when copying itself does not occur at all. Positive and negative bias together could allow faster spread of new adaptive behaviours. Also, it may be simpler to identify failure than success, especially across the species border.
Whether it is beneficial to use the behaviour of another species in decision-making at all, should depend on ecological overlap between the species, together with differences in opportunity and ability to accrue ‘knowledge’ (i.e. learned or innate adaptive behavioural responses) [4]. Residents should be copied more often than migrants, because more sedentary animals should have more and better knowledge relevant to the local conditions [18]. Furthermore, resident species tend to be (at least in birds) more innovative than migratory species [19], hence being more likely to possess new, locally adaptive behaviours. Notably, responding to the behaviour of another species need not, and perhaps even should not, depend on the ecological relevance of the behaviour. This is because new behaviours are by definition unknown to the observer and the ecological linkage that may make them locally relevant is often obscure. For example, copying or rejecting a novel type of breeding site could eliminate nest predation, or it might be utterly irrelevant, but the observer has no way to discern that. Irrelevant (or even harmful) behaviours may often get copied, and while they can be discarded with later personal experience, this further intensifies the need to be discriminating in whom to copy.
Migratory pied flycatchers (Ficedula hypoleuca) have a partially overlapping ecology with resident great tits (Parus major) and blue tits (Cyanistes caeruleus) during the nesting period in terms of predators, food and nest-site requirements [20], but the tits initiate breeding earlier. Many migratory passerines settle near to tits [21,22], and pied flycatchers have been shown to derive fitness benefits, such as larger clutches and bigger offspring [23] while the tits may suffer [24] from the association. Video evidence and frequent occurrence of killed flycatcher individuals in nest-boxes occupied by tits [25,26] show that pied flycatchers (and collared flycatchers F. albicollis) actively inspect nest boxes occupied by tits. These features of their co-occurrence suggest that copying tits could well be adaptive for pied flycatchers. Seppänen & Forsman [3] showed that late-arriving pied and collared flycatchers adopted novel nest-site preferences that were exhibited by all tits of a forest patch.
Here, we test with a field experiment whether observing the nest-site choice of a tit tutor influences the choice of a pied flycatcher female as predicted (here ‘tutor’ refers to inadvertent demonstration of a behaviour, not teaching). The experimental design (figure 1) seeks to remove, randomize or control other factors of pied flycatcher nest-site choice to isolate the effects of observing choices of tits. We portrayed an apparent tutor choice between two novel, neutral, alternative nest-site characteristics and presented a forced choice between these alternatives for arriving flycatcher females. Each female observed one breeding tit pair exhibiting the new trait, and we tested whether natural variation in the number of tutor offspring influenced observer choices as predicted: choices should more often match with tutors with a higher fitness correlate [11,12,14], while behaviour of tutors with the lowest fitness correlate should be actively rejected.
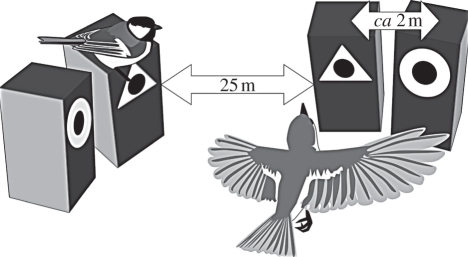
Figure 1.
Experimental setup. A symbol was randomly assigned to the tit nest, and the alternative symbol was attached to the adjacent empty box. These two boxes portray tutor choice, and tit territoriality prevents settling there. Two empty boxes with the alternative symbols were offered for arriving flycatchers approximately 25 m away, adjacent to each other. The two boxes fall within the small territory defended by male pied flycatchers, forcing the female to choose in which to build its nest. The tutor nest and its accompanying empty box were always visible from the flycatcher boxes. Replicate setups were at least a kilometre apart to ensure independence.
2. Material and methods
(a). Setup of the experiment
The experiment replications were conducted in Finnish mixed and coniferous forests near the city of Jyväskylä in 2007 and 2008 (separate areas JY07 and JY08), Oulu in 2008 (OU08), and in Latvian pine plantation forests near the city of Daugavpils in 2009 (DA09). Nest boxes for tits were distributed during winter along small roads throughout the study areas, and tits were allowed to settle freely. Almost all of the pairs were great tits (the data contain two coal tit (Parus ater) and five blue tit tutor nests). At the occupied boxes, we randomly assigned and attached at the nest-box entrance either a white circle or a triangle (7.5 cm diameter or sides). The front of the boxes was painted black to contrast with the white plastic symbols. On the nearest similar tree (2–6 m away), we put an empty nest-box with the opposite symbol, seeking to create the appearance that the tit pair had made a choice between the two alternatives. Boxes were so close that tits aggressively defend both, preventing flycatchers from settling in the vacant box. Consequently, none of these was occupied. At approximately 25 m distance from this first pair, we placed two empty boxes for the arriving flycatchers, facing the first pair, on adjacent similar trees (2–6 m apart), with the alternative symbols randomly assigned to left and right. These four boxes comprised one setup. The setups were at least a kilometre apart, to minimize the chances of flycatcher females observing symbols outside their own experimental setup. The areas surrounding the setups did contain other tit nests in natural cavities and old nest boxes. While these were not manipulated or monitored, flycatcher females probably visited them.
(b). Measurements
Boxes were inspected every second day, and flycatcher female choice and the date of choice were determined by the appearance of nest material. We measured the number of tit offspring (eggs or hatchlings) on the day of flycatcher choice. Two observations from the Finnish dataset were discarded as it was uncertain whether the tit nest was active when the flycatcher choice was made. We measured 34 flycatcher choices in JY07, 20 in JY08 and 44 in OU08 and 57 in DA09.
(c). Analyses
We fitted logistic regression to flycatcher choice match with the tit, explained by the number of tit offspring on the day of choice, the type of symbol on tit nest-box, study area and the interaction of offspring number with study area. As it was evident that the response in DA09 was stronger than in the Finnish areas, while those three were similar to each other, we analysed the Latvian and Finnish data separately. In subsequent analyses, models containing each of the possible two-way interactions were also fit in turn and compared with a simpler model with a likelihood ratio test (LRT), but none of the interactions were included as p ≫ 0.1; the effects of interactions are nonetheless included in graphical presentation of the fits in figure 2 to show repeatability of the response despite moderate differences in the constant term across study areas (Finland) and between symbol types (Latvia). We report LRT two-tailed p-values, and when the parameter estimate is significantly different from zero, the parameter estimate and its 95% confidence interval (CI).
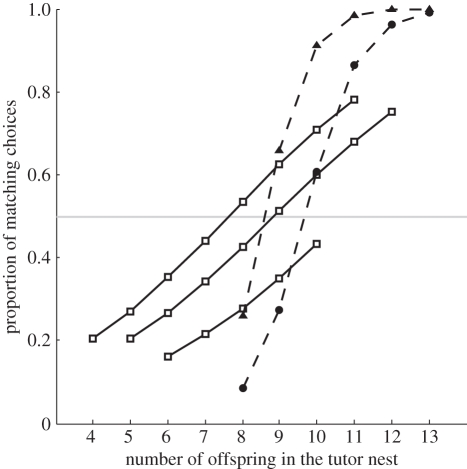
Figure 2.
Proportion of flycatcher choices matching with the portrayed tit tutor choice between two novel symbols (circle or triangle attached at nest-box entrance), as a function of the number of offspring in the heterospecific tutor nest. Lines show logistic regression fits at observed values of offspring number. Solid lines with open squares are for the three Finnish study areas (from top to bottom: OU08, JY07 and JY08); the type of symbol chosen by the tutor had negligible effect and is not included in the fits, but the effects of study area are included to show similarity of slope despite moderately (but non-significantly) differing constants. Dashed lines with black symbols (triangle and circle) are for the one Latvian study area (DA09); the fit includes the effect of the type of symbol (triangle or circle) attached on the tit tutor nest and responses are shown separately for the symbol types, to show significant flycatcher bias towards the triangle. However, the response slopes are very similar for triangles and circles.
3. Results
The probability of a flycatcher female choosing a nest site with a symbol matching the tit's was clearly positively dependent on the number of tit offspring on the day of choice (figure 2; Finland: log odds-ratio per offspring = 0.364 (0.089, 0.670), LRT: n = 98, χ2 = 6.86, p = 0.009; Latvia: log odds-ratio per offspring = 1.697 (0.746, 2.648), LRT: n = 57, χ2 = 28.44, p ≪ 0.001).
In particular, in all study areas flycatchers tended to choose the opposite symbol when tit tutor fitness was very low (figures 2 and 3). If the choices of poor tutors would have been merely ignored by the flycatchers, the proportion of matching choices among these setups should have been around the null expectation of 0.5. Instead, the proportion of matching choices was consistently less than 0.25 when the tutor fitness was lowest.
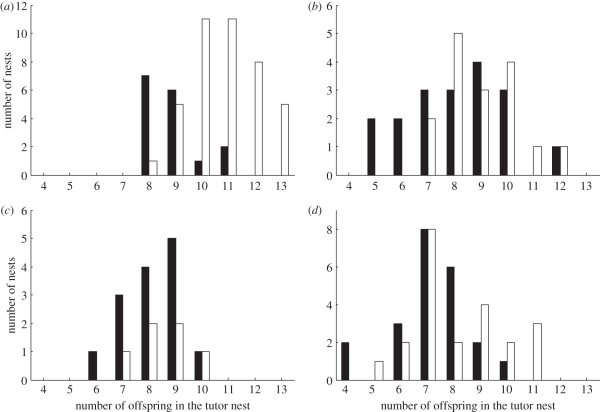
Figure 3.
The choices of pied flycatcher females in the four study areas (a) Daugavpils 2009; (b) Jyväskylä 2007; (c) Jyväskylä 2008 and (d) Oulu 2008. White bars denote number of pied flycatcher nests in the nestboxes with a symbol matching the symbol on the tit tutor's nest-box, black bars denote nests in the nestboxes with an opposite symbol. X-axis shows the number of offspring in the tit tutor nest on the day when presence of nest material indicated flycatcher choice.
The response was very similar between the three areas in Finland, with almost identical slopes and moderately (but non-significantly) differing constants. The response in DA09 was stronger and shifted towards larger numbers of offspring compared with the Finnish areas (figure 2). The type of symbol on the tutor nest had no effect on flycatcher response slope (LRT, model including the interaction compared with model with main effects only: n = 57, χ2 = −0.710, p = 0.399), but the constant term differed between the symbols in DA09, indicating more matching choices when the tutor nested in a triangle (log odds-ratio circle per triangle = −1.887 (−3.712, −0.062), LRT: n = 57, χ2 = 4.942, p = 0.026).
4. Discussion
Results demonstrate that flycatchers have a strong response to tit tutor fitness when forced to choose between two novel, arbitrary and neutral behaviours, without direct reward or penalty for either the heterospecific tutors or themselves. It is evident that rejection and adoption of a heterospecific's behaviour, biased by the observed correlate of tutor fitness, can indeed occur in the wild. We believe that this may be common when a species with better local knowledge (be it learned or innate) and sufficient ecological overlap is available [4].
Because tutor breeding was unmanipulated and all birds were free, we cannot conclusively exclude the possibility that flycatcher females responded to some other correlate of tit fitness (such as tit parent characteristics), or that some characteristic of the flycatcher female or environment (that then affected rejection/copying) covaried with the number of tit offspring on the day of flycatcher choice. However, a response to some covarying factor would be unlikely to result in almost identical response slopes observed in the three Finnish study areas (figure 2), unless it was almost perfectly correlated with tit offspring number on the day of flycatcher choice. Where data were available (OU08), we tested for (LRT, model including the confounding factor compared with a model with tit offspring number only) but did not find any effect of tit age (females: n = 40, χ2 = 0.423, p = 0.516; males: n = 40, χ2 = 0.007, p = 0.932) or weight (females: n = 40, χ2 = –2.341, p = 0.126; males: n = 40, χ2 = –0.585, p = 0.444) on flycatcher choice. Nor did the age (n = 42, χ2 = –0.371, p = 0.543) or weight (n = 41, χ2 = –0.464, p = 0.496) of the flycatcher female itself, or date of its choice (n = 44, χ2 = –0.964, p = 0.326), have an effect on the choices. Thus, the direct response to the most conspicuous correlate of tutor fitness—the number of offspring in the nest—is the most parsimonious interpretation for the observed relationship.
Responding to quantitative cues, perhaps even counting, is likely to occur widely among birds (see discussion in [27]), through visual or tactile stimuli. But discriminating response to observed number of offspring also requires some standard of comparison by which flycatcher females were able to respond differently to poor- and high-fitness tutors. The way this is achieved is unknown and merits further research, but two mechanisms are plausible. First, flycatcher females probably inspect several tit nests in the area, and may compare the number of offspring between those—a nest with higher value than the surrounding nests would be perceived as having high fitness, regardless of the absolute number of offspring. This would also explain some of the variation, and the slightly different constants but similar slopes of the responses between Finnish study areas, because average tit breeding success (and hence the standard employed by flycatchers) varies both within and between study areas, and between years. Second, the response may hinge on a genetically determined threshold to sensory stimuli. Because it is plausible that the response to heterospecifics is derived from an originally intraspecific behaviour, and because pied flycatcher clutch size varies relatively little around its median, flycatchers might have a genetically determined standard for the sensory stimuli corresponding to ‘average’ number of offspring in a conspecific nest, and modification of this for heterospecifics. However, because study areas did differ, and sampling alone would seem to require considerable effort, we suspect that both mechanisms occur so that a genetically determined threshold is modified through sampling.
An earlier experiment where all tit nests of a forest patch were assigned the same symbol while an empty nest with the opposite symbol was placed adjacent to each [3] to portray patch-wide preference among the tits, found that late-arriving flycatchers were more prone to copy the apparent choice of the tits than the ones arriving early. Because that experiment involved a large number of tutors, it is not directly comparable to the single-tutor design reported here, but the patterns may be connected. Discriminative copying as reported here could partially explain why later-arriving flycatchers were more likely to copy the tits in Seppänen & Forsman [3], because later-arriving flycatchers were more likely to observe only completed, larger tit clutches. The signal for earlier-arriving flycatchers was perhaps diluted by the presence of small, incomplete clutches.
Remarkably, flycatchers rejected the preference of a poor tutor, instead of merely ignoring it and choosing randomly. The thus far proposed copying strategies [14] have implicitly assumed that the only alternative to copying is to ignore the observed behaviour altogether. As this experiment demonstrates, ‘not-copying’ does not necessarily equate to ignoring the observed behaviour. Active rejection of behaviours of poor individuals can facilitate decision-making by reducing the set of alternatives to choose from, thus reducing uncertainty. Especially when the number of alternatives to choose from is small, being able to discard even one of the alternatives provides considerable advantage. In the case of just two alternatives (a binary choice), rejection of the alternative exhibited by a poor individual leaves just one alternative to be adopted.
In concert with earlier discoveries [3], this study indicates that flycatchers perceive [25] and make decisions [28] based on the presence, behaviour and fitness of tits. This ability may well be an extension of adaptations originally evolved in conspecific context. For example, observing lowered numbers of conspecific offspring at a patch increases collared flycatcher emigration and decreases immigration [6]. Generalizations of similar intricate strategies should abound across the taxonomic and contextual diversity [4] of decision-making influenced by the behaviour of heterospecifics.
If common, rejecting and copying behaviour of individuals of other species based on observable fitness can have important consequences for niche overlap and partitioning, and consequently species co-existence and behavioural similarity. One of the central tenets of evolutionary ecology is that coexistence of species using shared resources results in, and requires, character displacement [29–31]. Ecological overlap between species should be minimal, and those individuals that share the most traits with another species would tend to be in the margins of their own species' niche, suffer from competition, and consequently have generally low fitness. By contrast, interspecific copying should directly increase the number of shared behaviours [3], and using other species in decision-making in general could select for character convergence [4]. Owing to discriminative copying, ecological and behavioural overlap would be highest between best-performing individuals of both species and most common when resources are abundant. Conversely, the active rejection of behaviours of poor individuals can help avoiding costly errors, and may enhance character displacement when resources are scarce.
In conclusion, it is conceivable that a behaviour currently present in a population of given species may sometimes originate from and continue to be influenced by opportunities to observe individuals of another species. How these invisible interactions affect individuals, populations and communities [4]—and how human activity impacts these dynamics [32]—may become an important frontier in evolutionary, behavioural and conservation ecology.
Acknowledgements
Birds were caught and handled under national ringing licences and study protocols comply with Finnish and Latvian legislation.
We thank Tiina Kananoja, Tatjana Krama and Mikus Abolins-Abols for help in fieldwork, and technical staff at University of Jyväskylä and University of Oulu Biological Research Facility for providing material support. We also thank Tuomo Jaakkonen, Wiebke Schuett and Robert Thomson for comments. The Academy of Finland funded J.T.S. (no. 125720), J.T.F. (no.122665), and M.M. (no. 115560) and T.S. received a grant from Societas Biologica Fennica Vanamo.
References
- 1.Lefebvre L., Templeton J., Brown K., Koelle M. 1997. Carib grackles imitate conspecific and Zenaida dove tutors. Behaviour 134, 1003–1017 10.1163/156853997X00368 (doi:10.1163/156853997X00368) [DOI] [Google Scholar]
- 2.Coolen I., Van Bergen Y., Day R. L., Laland K. N. 2003. Species difference in adaptive use of public information in sticklebacks. Proc. R. Soc. Lond. B 270, 2413–2419 10.1098/rspb.2003.2525 (doi:10.1098/rspb.2003.2525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seppänen J.-T., Forsman J. T. 2007. Interspecific social learning: novel preference can be acquired from a competing species. Curr. Biol. 17, 1248–1252 10.1016/j.cub.2007.06.034 (doi:10.1016/j.cub.2007.06.034) [DOI] [PubMed] [Google Scholar]
- 4.Seppänen J.-T., Forsman J. T., Mönkkönen M., Thomson R. L. 2007. Social information use is a process across time, space and ecology, reaching heterospecifics. Ecology 88, 1622–1633 10.1890/06-1757.1 (doi:10.1890/06-1757.1) [DOI] [PubMed] [Google Scholar]
- 5.Goodale E., Beauchamp G., Magrath R. D., Nieh J. C., Ruxton G. D. 2010. Interspecific information transfer influences animal community structure. Trends Ecol. Evol. 25, 354–361 10.1016/j.tree.2010.01.002 (doi:10.1016/j.tree.2010.01.002) [DOI] [PubMed] [Google Scholar]
- 6.Doligez B., Danchin E., Clobert J. 2002. Public information and breeding habitat selection in a wild bird population. Science 297, 1168–1170 10.1126/science.1072838 (doi:10.1126/science.1072838) [DOI] [PubMed] [Google Scholar]
- 7.Fragaszy D. M., Perry S. 2003. The biology of traditions: models and evidence. Cambridge, UK: Cambridge University Press [Google Scholar]
- 8.Danchin E., Giraldeau L.-A., Valone T. J., Wagner R. H. 2004. Public information: from nosy neighbors to cultural evolution. Science 305, 487–491 10.1126/science.1098254 (doi:10.1126/science.1098254) [DOI] [PubMed] [Google Scholar]
- 9.Bonnie K. E., Earley R. L. 2007. Expanding the scope for social information use. Anim. Behav. 74, 171–181 10.1016/j.anbehav.2006.12.009 (doi:10.1016/j.anbehav.2006.12.009) [DOI] [Google Scholar]
- 10.Hoppitt W., Laland K. N. 2008. Social processes influencing learning in animals: a review of the evidence. Adv. Stud. Behav. 38, 105–165 10.1016/S0065-3454(08)00003-X (doi:10.1016/S0065-3454(08)00003-X) [DOI] [Google Scholar]
- 11.Boyd R., Richerson P. J. 1985. Culture and the evolutionary process. Chicago, IL: University of Chicago Press [Google Scholar]
- 12.Henrich J., Gil-White F. J. 2001. The evolution of prestige: freely conferred status as a mechanism for enhancing the benefits of cultural transmission. Evol. Hum. Behav. 22, 165–196 10.1016/S1090-5138(00)00071-4 (doi:10.1016/S1090-5138(00)00071-4) [DOI] [PubMed] [Google Scholar]
- 13.Giraldeau L.-A., Valone T. J., Templeton J. J. 2002. Potential disadvantages of using socially acquired information. Phil. Trans. R. Soc. Lond. B 357, 1559–1566 10.1098/rstb.2002.1065 (doi:10.1098/rstb.2002.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laland K. N. 2004. Social learning strategies. Learn. Behav. 32, 4–14 [DOI] [PubMed] [Google Scholar]
- 15.Kendal R. L., Coolen I., Bergen Y. V., Laland K. N. 2005. Tradeoffs in the adaptive use of social and asocial learning. Adv. Stud. Behav. 35, 333–379 10.1016/S0065-3454(05)35008-X (doi:10.1016/S0065-3454(05)35008-X) [DOI] [Google Scholar]
- 16.Dugatkin L. A., Godin G. J. 1993. Female mate copying in the guppy (Poecilia reticulata): age-dependent effects. Behav. Ecol. 4, 289–292 10.1093/beheco/4.4.289 (doi:10.1093/beheco/4.4.289) [DOI] [Google Scholar]
- 17.Duffy G. A., Pike T. W., Laland K. N. 2009. Size-dependent directed social learning in nine-spined sticklebacks. Anim. Behav. 78, 371–375 10.1016/j.anbehav.2009.05.015 (doi:10.1016/j.anbehav.2009.05.015) [DOI] [Google Scholar]
- 18.Mönkkönen M., Härdling R., Forsman J. T., Tuomi J. 1999. Evolution of heterospecific attraction: using other species as cues in habitat selection. Evol. Ecol. 13, 91–104 [Google Scholar]
- 19.Sol D., Lefebvre L., Rodriguez-Teijeiro J. D. 2005. Brain size, innovative propensity and migratory behaviour in temperate Palaearctic birds. Proc. R. Soc. B 272, 1433–1441 10.1098/rspb.2005.3099 (doi:10.1098/rspb.2005.3099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundberg A., Alatalo R. V. 1992. The pied flycatcher. London, UK: T & AD Poyser [Google Scholar]
- 21.Mönkkönen M., Helle P., Niemi G. J., Montgomery K. 1997. Heterospecific attraction affects community structure and migrant abundances in northern breeding bird communities. Can. J. Zool. 75, 2077–2083 10.1139/z97-842 (doi:10.1139/z97-842) [DOI] [Google Scholar]
- 22.Thomson R. L., Mönkkönen M., Forsman J. T. 2003. Positive interactions between migrant and resident birds: testing the heterospecific attraction hypothesis. Oecologia 134, 431–438 [DOI] [PubMed] [Google Scholar]
- 23.Forsman J. T., Seppänen J.-T., Mönkkönen M. 2002. Positive fitness consequences of interspecific interaction with a potential competitor. Proc. R. Soc. Lond. B 269, 1619–1623 10.1098/rspb.2002.2065 (doi:10.1098/rspb.2002.2065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forsman J. T., Thomson R. L., Seppänen T. 2007. Mechanisms and fitness effects of interspecific information use between migrant and resident birds. Behav. Ecol. 18, 888–894 10.1093/beheco/arm048 (doi:10.1093/beheco/arm048) [DOI] [Google Scholar]
- 25.Forsman J. T., Thomson R. L. 2008. Evidence of information collection from heterospecifics in cavity-nesting birds. Ibis 150, 409–412 10.1111/j.1474-919X.2007.00773.x (doi:10.1111/j.1474-919X.2007.00773.x) [DOI] [Google Scholar]
- 26.Merilä J., Wiggins D. A. 1995. Interspecific competition for nest holes causes adult mortality in the collared flycatcher. Condor 97, 445–450 10.2307/1369030 (doi:10.2307/1369030) [DOI] [Google Scholar]
- 27.Odell N. S., Eadie J. M. 2010. Do wood ducks use the quantity of eggs in a nest as a cue to the nest's value? Behav. Ecol. 21, 794–801 10.1093/beheco/arq055 (doi:10.1093/beheco/arq055) [DOI] [Google Scholar]
- 28.Forsman J. T., Hjernquist M. B., Taipale J., Gustafsson L. 2008. Competitor density cues for habitat quality facilitating habitat selection and investment decisions. Behav. Ecol. 19, 539–545 10.1093/beheco/arn005 (doi:10.1093/beheco/arn005) [DOI] [Google Scholar]
- 29.Brown W. L., Wilson E. O. 1956. Character displacement. Syst. Zool. 5, 49–65 10.2307/2411924 (doi:10.2307/2411924) [DOI] [Google Scholar]
- 30.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press [Google Scholar]
- 31.Losos J. B., Ricklefs R. E. 2009. Adaptation and diversification on islands. Nature 457, 830–836 10.1038/nature07893 (doi:10.1038/nature07893) [DOI] [PubMed] [Google Scholar]
- 32.Holt R. D. 2007. The unraveling of nature's information webs: the next depressing frontier in conservation? Isr. J. Ecol. Evol. 53, 229–236 10.1560/IJEE.53.3.229 (doi:10.1560/IJEE.53.3.229) [DOI] [Google Scholar]