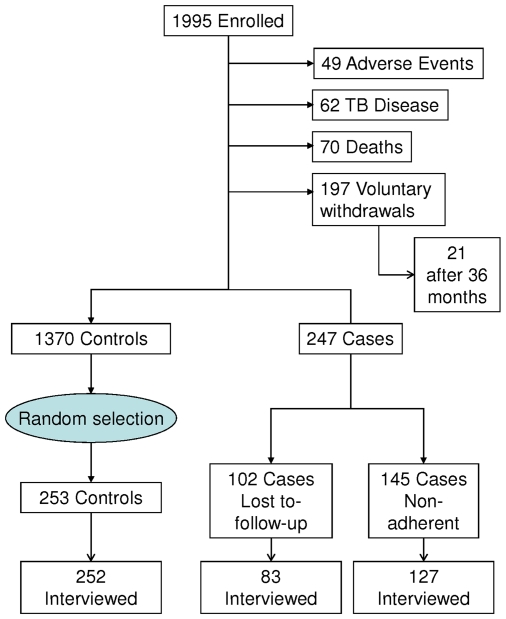
Figure 1. Derivation of cases and controls for the adherence sub-study from the cohort of HIV-infected persons enrolled in the Botswana Isoniazid Preventive Therapy Trial, 2004–2008.
Case-Non-adherent was defined as not taking the study medication due to unwillingness to take any more study medication but continuing to attend quarterly visits and seen at the last expected visit. Case-LosT to follow-up was defined as a participant who was still expected to take the study medication and receive monthly medication refills but missed the last visit by≥60 days. A control was defined as a participant who continued to be on study medication and was last seen within the expected visit window which was 7 days early or 14 days late in the 30-day study month. The median number of days since the last visit of those cases who were lost to follow-up was 396 days (range 91–1196), and 48 days (range 6–116) for the cases who were non-adherent. As the sub-study was conducted between 10/2008 AND 4/2009, 21 PARTICIPANTS HAS ALREADY COMPLETED THE RQUIRED 36 MONTHS OF OBSERVATION AND HAD VOLUNTARILY WITHDRAWN.