Abstract
This review describes some of the physiological effects of recombinant human erythropoietin (EPO) in healthy humans. At the blood level EPO increases the arterial O2 content not only by increasing red blood cell volume, but also by an equally important decrease in plasma volume. Well before that, EPO causes a prompt decrease in plasma levels of renin and aldosterone. Renal clearance studies suggest that EPO decreases renal proximal tubular reabsorption rate leading to activation of the tubuloglomerular feedback mechanism and a fall in glomerular filtration rate. Thus, treatment with EPO may result in suppression of endogenous EPO production through a decrease in intrarenal oxygen consumption. EPO elevates the arterial blood pressure even in healthy subjects. The receptor for EPO is present in many tissues. However, the functional effects of EPO in the skeletal muscle seem limited, and although it has been speculated that non-erythropoietic effects of EPO (angiogenesis, shift in muscle fibre types, cognitive effects) may be responsible for the increase in exercise performance, this has not been confirmed. EPO-induced haemodynamic effects call for careful monitoring during the administration period. The metabolic, hormonal and renal effects of EPO do not seem to range beyond physiologically acceptable limits and are reversible. Taken together, EPO seems safe to use for experimental purposes in healthy volunteers.
Carsten Lundby qualified as a physiologist at University of Southern Denmark, and completed his PhD under guidance of Prof. Bengt Saltin at Copenhagen Muscle Research Center, during which a number of studies on functions of erythropoietin in humans were initiated. After one year post doc in Zürich he became assistant professor (and later also associated professor) at Dept of Sport Science at University of Aarhus. Current research group in Center for Integrative Human Physiology (ZIHP) at University of Zürich focuses on oxygen transport and utilization in humans from cellular to systemic level. Niels Vidiendal Olsen studied medicine at Copenhagen University. A chief anaesthetist and educator at Copenhagen University Hospital, he regularly contributes reviews and articles in international journals. His work includes participation in high altitude and exercise studies and he is the author of several papers on the pharmacology of erythropoietin.
 |
During the last decade many new and exciting functions have been attributed to erythropoietin (EPO), and many of these are related to non-erythropoietic effects (Jelkmann, 2007). This review outlines some of the most physiologically relevant functions and mechanisms of EPO with an emphasis on studies conducted in healthy subjects. Several functions of EPO, i.e. inhibition of inflammation and apoptosis, anti-oxidant effects and stimulation of angiogenesis, may be of potential use in the treatment of pathological conditions but to address the clinical aspects is beyond the scope of the present review. Along with news of potentially beneficial effects attributed to EPO, reports have also been advanced indicating that the mortality and morbidity rates are increased in some patient groups when treated with EPO (Phrommintikul et al. 2007; Ehrenreich et al. 2009; Pfeffer et al. 2009; Dicato & Plawny, 2010). Thus, the question arises whether it can be considered safe to conduct studies with EPO in normal subjects.
The mechanisms by which EPO increases haematocrit and O2 transport capacity
In healthy individuals with haematocrit values in the normal range, injections of around 60 IU kg−1 one to three times per week for 4–14 weeks increase the haematocrit to just below 50% (Table 1). The main mechanism for the increase in haematocrit was long thought to rely exclusively on augmentations in red cell mass, but it was demonstrated that EPO also decreases plasma volume (Lundby et al. 2007). In fact the relative contributions of an augmented red cell mass and a concomitant decrease in plasma volume to the increase in blood haemoglobin levels were almost equal over a 13 weeks period. This response may require a normal renal function and may not be observed in patients with kidney diseases. The plasma volume-reducing effects of EPO was confirmed in other studies (Lundby et al. 2008a; Olsen et al. 2010). It is noteworthy that the change in plasma volume preceded the increase in red blood cell volume. A preliminary study suggested that the newly formed erythrocytes undergo apoptosis once EPO stimulation is stopped, so that the EPO-induced increase in red cell mass does not last the expected 120 days (Chang et al. 2009). In contrast, however, and based on direct measures of red cell mass following the termination of EPO stimulation, it would appear that haemoglobin concentration [Hb] is normalized rather rapidly due to a restoration in plasma volume without an effect on red cell mass (Lundby et al. 2008a; Olsen et al. 2010). This implies that although [Hb] and haematocrit are promptly returned toward baseline values after the EPO administration period, red cell O2 transport capacity may still remain elevated. How long red cell mass remains elevated following EPO treatment is at present unknown.
Table 1.
Effects of EPO injections in healthy subjects on haematocrit (htc), haemoglobin concentration ([Hb]), body weight and arterial blood pressure
| Injection period 1 | Injection period 2 | Iron supplementation | Pre-htc (%)/Hb (g dl−1) | Post-htc (%)/Hb (g dl−1) | Changes in body mass | Blood pressure (mmHg) | Reference |
|---|---|---|---|---|---|---|---|
| 3/week for 5 weeks @ 50 IU kg−1 | 3/week for 3 weeks @ 20 IU kg−1 | Oral supplement | 41.9/14.2 | 48.6/16.8 | No change | — | (Russell et al. 2002) |
| 3/week for 5 weeks @ 50 IU kg−1 | 3/week for 3 weeks @ 20 IU kg−1 | Intravenous infusion | 43.6/15.0 | 49.1/17.1 | No change | — | (Russell et al. 2002) |
| 10/3 weeks @ 60 IU kg−1 | 1/week for 9 weeks @ 60 IU kg−1 | Oral supplement | 42.0/14.2 | 49.0/17.1 | No change | +9 | (Lundby et al. 2007) |
| Daily for 26 days @ 50 IU kg−1 | — | Oral supplement | 44.4/15.1 | 47.9/15.9* | No change | — | (Audran et al. 1999) |
| 3/week for 4 weeks @ 60–77 IU kg−1 | — | Oral supplement | 42.7% | 50.8%* | No change | — | (Birkeland et al. 2000) |
| 3/week for 6 weeks @ 20–40 IU kg−1 | — | — | 44.5% | 49.7% | No change | No change§ | (Berglund & Ekblom, 1991) |
| 1/week for 4 weeks @ 150 IU kg−1 | — | No supplement | 43/15.8 | 49/16.9 | No change | No change | (Wilkerson et al. 2005) |
| Every second day/2 weeks @ 65 IU kg−1 | 1/week for 2 weeks @ 65 IU kg−1 | No supplement | 43.9% | 46.8% | No change | +4 | (Lundby et al. 2008a) |
| 3/week for 4 weeks @ 50 IU kg−1 | — | Oral supplement | 44.4/14.6 | 48.1/16.0 | No change | — | (Connes et al. 2003) |
| 12/26 days @ 50 IU kg−1 | — | Intramuscular injections | 14.8 g dl−1 | 15.9 g dl−1 | No change | — | (Parisotto et al. 2000) |
| 12/26 days @ 50 IU kg−1 | — | Oral supplementation | 15.0 g dl−1 | 16.6 g dl−1 | No change | — | (Parisotto et al. 2000) |
The protocol was discontinued for two subjects because htc increased to over 50%. §Blood pressure was increased during submaximal exercise.
The influence of EPO on blood pressure
When treatment with EPO in anaemic states became widely appreciated it was recognized that long-term administration of EPO in patients with chronic kidney disease provokes arterial hypertension. The possible mechanisms for this effect, which occurs independently of EPO's haematopoietic effect, have been recently reviewed (Krapf & Hulter, 2009). As shown in Table 1, however, in healthy subjects EPO injections sufficient to increase the haematocrit apparently do not increase the blood pressure. In these studies, blood pressure was usually not the main target investigated and was assessed by the use of sphygmomanometry. In contrast to these studies, the few studies assessing blood pressure by intra-arterial pressure transducers reported a small increase in resting values (Lundby et al. 2007). During exercise, haemodilution following EPO treatment may revert the augmented blood pressure response (Lundby et al. 2008c). This suggests a significant role of enhanced blood viscosity in EPO-induced hypertension during exercise. EPO-induced hypertension may, however, also be unrelated to viscosity, as acute injections of high EPO doses (30,000 IU day−1 for 3 days) increases both resting arterial blood pressure and the blood pressure response to exercise in normal subjects (Rasmussen et al. 2010). EPO treatment with prolonged low-dose administration or high doses for 3 days both induced an increase in total peripheral resistance, and, as assessed by transcranial Doppler measurements and cerebral arterio-venous differences in oxygen content, this vasoconstriction also included the cerebrovascular circulation. The mechanisms remain unclear, but may involve EPO-induced release of endothelin (Bode-Böger et al. 1996) and inhibition of eNOS-mediated production of NO (Wang & Vaziri, 1999; Scalera et al. 2005). EPO has also been shown to possess direct vasoconstrictive effects in isolated renal resistance vessels (Heidenreich et al. 1991) and human placental arteries in vitro (Resch et al. 2003). Thus, in normal subjects both long-term administration of low doses of EPO and short-term bouts of high-dose EPO may produce arterial hypertension and intracerebral vasoconstriction.
Metabolism: a functional regulating role of EPO?
In haemodialysis patients it was demonstrated that EPO treatment over a one year period lowered the body mass index (BMI) from 22.7 to 22.1 if food intake was not increased (Allegra et al. 1997). Because resting metabolic rate is not altered after prolonged EPO treatment in healthy individuals (Lundby et al. 2008c), and since leptin, which is secreted by the adipose tissue in proportion to the fat stores (Rosenbaum & Leibel, 1999), is decreased after 3–6 months of EPO treatment (Kokot et al. 1998) it could be that EPO induced a reduction in BMI by a fat tissue-reducing effect. In this regard, supra-physiological EPO doses may protect against obesity in mice if on a high fat diet (Hojman et al. 2009). Furthermore, trials in diabetic, chronic renal disease patients have shown that EPO improves several metabolic parameters including fasting glucose level and insulin sensitivity as measured by euglycaemic hyperinsulinaemic clamp and intravenous glucose tolerance test (Borissova et al. 1993; Allegra et al. 1996). These findings are, however, not supported by experiments where very high EPO doses (400 IU kg−1) were applied in combination with insulin clamp but did not show effects on glucose or fat metabolism at the skeletal muscle level in healthy humans (Christensen et al., unpublished). It was concluded that EPO injections were not accompanied by distinct alteration in the pattern of substrate metabolism or insulin sensitivity. None of the studies listed in Table 1 have confirmed the early reports of EPO-induced changes in body composition, and taken together, EPO in normal subjects does not seem to have major metabolic effects.
Skeletal muscle
Recently the receptor for EPO was reported in biopsies obtained from human skeletal muscle (Lundby et al. 2008b; Rundqvist et al. 2009). Whether this also implies a functional role or not has been questioned (Sinclair et al. 2010). Nevertheless, it seems that EPO injections in healthy volunteers may affect the human skeletal muscle both direct and indirectly. With acute injections of 15,000 IU the mRNA levels of VEGF, HIF-1α, IGF, ferroportin, MyoD and myogen remained unchanged in biopsies obtained 2, 4, 6 and 10 h after the EPO injections, whereas minor inductions were observed in myoglobin, epo-r, transferin receptor and MRF4 (Lundby et al. 2008b). Although this implies that EPO has at least some direct effects on the skeletal muscle, the physiological consequences of this seem very limited because long-term EPO treatment did not have any effect on the absolute number of capillaries, fibre-type distribution, cross-sectional area of the fibres (Lundby et al. 2008b), or on the majority of proteins involved in acid–base balance, except for a decrease in the Na+,K+-pump subunit α2 (Juel et al. 2007). Also, in the later study, no difference in muscle mass (or body composition) was reported following DEXA scans (Lundby et al. 2008b). EPO may, however, indirectly affect the human skeletal muscle. It has been hypothesized that EPO injections would cause skeletal muscle iron stores to decrease in order to make iron available for red cell formation (Robach et al. 2007). However, on the contrary, following injections with EPO, skeletal muscle accumulates iron despite systemic iron deficiency secondary to EPO-induced erythropoiesis and hepcidin-promoting suppression of iron store mobilization (Robach et al. 2009). The exact mechanism for this remains at present unknown.
The renin–angiotensin–aldosterone system and renal function
Production of endogenous EPO is regulated by the renin–angiotensin system (Dunn et al. 2007). When normal human subjects are given angiotensin II the plasma levels of EPO increase (Freudenthaler et al. 2000; Gossmann et al. 2001), and, conversely, inhibition of angiotensin-converting enzyme decreases plasma EPO (Pratt et al. 1992). The changes in EPO synthesis are probably mediated through an angiotensin II receptor-1-dependent pathway (Gossmann et al. 2001). We have shown the presence of a contra-productive pathway by which treatment with EPO promptly, and before any changes in haematocrit, blood volumes and blood pressure can be detected, down-regulates circulating levels of renin and aldosterone (Lundby et al. 2007; Olsen et al. 2010).
Concomitant clearance studies revealed that the EPO-induced decrease in renin and aldosterone was associated with a fall in glomerular filtration rate (GFR). Furthermore, lithium clearance, used as an index of proximal tubular outflow, increased indicating a 10–16% decrease in proximal tubular reabsorption of sodium and water (Olsen et al. 2010). Changes in end-proximal delivery of tubular fluid to the macula densa produce inverse changes in renin release (Briggs et al. 1990) and thus the suppression of plasma renin levels may be secondary to direct effects of EPO on proximal tubular reabsorption. In addition, a decrease in proximal tubular reabsorption activates the tubuloglomerular feedback mechanism causing a parallel decrease in GFR (Holstein-Rathlou, 1991). The molecular mechanisms for EPO's effect on the proximal tubule remain unknown but may involve inhanced release of renal endothelin-1 (Bode-Böger et al. 1996) which in low doses attenuates sodium reabsorption in the proximal tubule (Clavell et al. 1995). Tubular reabsorption of sodium is the main oxygen-consuming process in the kidney and around 70% of the filtered load is reabsorbed in the proximal tubule. By inhibiting proximal tubular reabsorption, which in turn results in rapid declines in GFR and renin/aldosterone levels, EPO directly reduces the major oxygen-consuming factor in the kidney, reduces the filtered load, and decreases angiotensin II- and aldosterone-dependent reabsorption in more distal nephron segments. The expected result will be an increase of the oxygen tension in the environment of renal EPO-producing cells, in this way initiating an appropriate signal for down-regulation of endogenous EPO synthesis when circulating levels of recombinant EPO are high (Fig. 1). In support of such a feedback system, evidence exists to indicate that prolonged administration of recombinant EPO results in a suppression of urinary excretion of endogenous EPO (Lasne et al. 2002), and also the renal effects of recombinant EPO fit well with the hypothesis that the kidney operates as a ‘critmeter’ to regulate the EPO synthesis and body haematocrit through the metabolic signal of renal tissue oxygen pressure (Donnelly, 2003).
Figure 1. How high levels of circulating recombinant EPO may result in suppression of endogenous EPO synthesis secondary to a decrease in intrarenal oxygen consymption, by intrinsic renal effects.
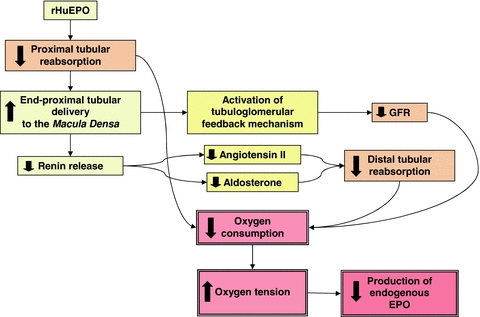
(1) EPO decreases reabsorption of sodium and fluid in the proximal tubule, thereby directly reducing the major oxygen-consuming process in the kidney; (2) increase in end-proximal tubular delivery to the macula densa decreases renin release and subsequent angiotensin II- and aldosterone-dependent reabsorption in more distal nephron segments; (3) decreased proximal tubular reabsorption activates the tubuloglomerular feedback mechanism producing a fall in GFR and reduction of the filtered load; (4) the resulting increase in renal oxygen partial pressure in the environment of interstitial fibroblast-like cells down-regulates the hypoxia-inducible factor-2-dependent production of endogenous EPO.
The reduction in plasma volume induced by EPO may be caused by natriuresis secondary to the hyporeninaemic hypoaldosteronism (Lundby et al 2007; Krapf & Hulter 2009). The renal sodium loss necessary to account for the associated decrease in plasma volume is small and perhaps the net effect of EPO is to cause a negative sodium balance during the treatment period.
Exercise capacity
Exercise performance is the result of multiple factors. Most studies investigating the effects of EPO on performance have evaluated maximal oxygen uptake (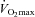 ). When haematocrit is increased from base values to around 50%,
). When haematocrit is increased from base values to around 50%, 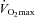 is increased by 8–12% (Audran et al. 1999; Birkeland et al. 2000; Russell et al. 2002; Thomsen et al. 2007; Lundby et al. 2008a,c; Robach et al. 2008). The performance-enhancing effects of EPO on
is increased by 8–12% (Audran et al. 1999; Birkeland et al. 2000; Russell et al. 2002; Thomsen et al. 2007; Lundby et al. 2008a,c; Robach et al. 2008). The performance-enhancing effects of EPO on 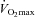 at different altitudes has been investigated and it was demonstrated that EPO provided the greatest advantage at medium altitudes (1500–2500 m) (Lundby & Damsgaard, 2006; Robach et al. 2008). One study has assessed the performance gains at submaximal exercise intensities, and demonstrated improvements of more than 50% in a time to exhaustion test (Thomsen et al. 2007). Exercise performance could be increased by factors other than those attributed to changes in red blood cell volume. In subjects haemodiluted isovolumically (Lundby et al. 2008c), i.e. restoring blood parameters to pre-EPO treatment values following 13 weeks of EPO treatment, however,
at different altitudes has been investigated and it was demonstrated that EPO provided the greatest advantage at medium altitudes (1500–2500 m) (Lundby & Damsgaard, 2006; Robach et al. 2008). One study has assessed the performance gains at submaximal exercise intensities, and demonstrated improvements of more than 50% in a time to exhaustion test (Thomsen et al. 2007). Exercise performance could be increased by factors other than those attributed to changes in red blood cell volume. In subjects haemodiluted isovolumically (Lundby et al. 2008c), i.e. restoring blood parameters to pre-EPO treatment values following 13 weeks of EPO treatment, however, 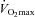 was similar to the values obtained prior to any injections. Since in this study the EPO dosages were probably too small to cross the blood–brain barrier, and since EPO induces the perception of superior physical fitness (Ninot et al. 2006), Rasmussen and co-workers (Rasmussen et al. 2010) injected 30,000 IU in three consecutive days to test whether EPO crossing the brain–blood barrier, and hence potentially interacting with the CNS, could increase submaximal exercise performance. Analysis of spinal fluid revealed a massive increase in EPO following the injections, and hence proved that EPO had in fact crossed the blood–brain barrier. Also in this study, however, no changes in exercise performance were noted, and surprisingly the rate of perceived exertion during the exercise became worse. Also in this regard, it was elegantly demonstrated that the O2 kinetics during the onset of exercise are not altered by EPO (Wilkerson et al. 2005), something that had been proposed a few years earlier (Connes et al. 2003). Thus, there is no evidence indicating that EPO should increase exercise performance by mechanisms other than increasing oxygen transport capacity. One could speculate that an increase in haemoglobin mass (nHb) may positively influence repeated sprint performance because of increased buffer capacity, but this remains unexplored.
was similar to the values obtained prior to any injections. Since in this study the EPO dosages were probably too small to cross the blood–brain barrier, and since EPO induces the perception of superior physical fitness (Ninot et al. 2006), Rasmussen and co-workers (Rasmussen et al. 2010) injected 30,000 IU in three consecutive days to test whether EPO crossing the brain–blood barrier, and hence potentially interacting with the CNS, could increase submaximal exercise performance. Analysis of spinal fluid revealed a massive increase in EPO following the injections, and hence proved that EPO had in fact crossed the blood–brain barrier. Also in this study, however, no changes in exercise performance were noted, and surprisingly the rate of perceived exertion during the exercise became worse. Also in this regard, it was elegantly demonstrated that the O2 kinetics during the onset of exercise are not altered by EPO (Wilkerson et al. 2005), something that had been proposed a few years earlier (Connes et al. 2003). Thus, there is no evidence indicating that EPO should increase exercise performance by mechanisms other than increasing oxygen transport capacity. One could speculate that an increase in haemoglobin mass (nHb) may positively influence repeated sprint performance because of increased buffer capacity, but this remains unexplored.
Cognitive function
Although EPO did not reduce the central fatigue during exercise (Rasmussen et al. 2010), evidence now exists to suggest that high doses modulate neural processing and may improve cognitive function 3 and 7 days after the administration of EPO (Miskowiak et al. 2008a,b). 40,000 IU of intravenously administered EPO lead to better performance in healthy subjects in a test of verbal fluency 7 days after injection of EPO (Miskowiak et al. 2008a). Concomitant use of functional magnetic resonance imaging demonstrated that EPO has an enduring effect on neurotrophic signalling in neural networks often affected in neuropsychiatric diseases (Miskowiak et al. 2008a,b;). Also a beneficial effect of 12 weekly injections of 40,000 IU of EPO on cognitive function has been reported in patients with schizophrenia (Ehrenreich et al. 2007).
Is it safe to conduct studies with EPO in healthy volunteers?
EPO has emerged as a pleiotropic substance with multiple targets of action. The question arises whether the newly discovered effects of EPO hamper its use in physiological research studies beyond the risk associated with known haematopoietic effects. Of greatest concern is the fact that EPO may increase arterial blood pressure even in healthy subjects. It is noteworthy also that short-term administration of high EPO doses may cause peripheral and intracerebral vasoconstriction. Although the effect is small it may call for careful monitoring of the blood pressure during the administration period. The metabolic, hormonal and renal effects of EPO are small and do not seem to range beyond physiologically acceptable limits. Furthermore, the effects observed thus far have been fully reversible. Taken together, the new evidence may call for more information to subjects participating in EPO trials but does not push the stop button.
References
- Allegra V, Martimbianco L, Vasile A. Lipid and apolipoprotein patterns during erythropoietin therapy: roles of erythropoietin, route of administration, and diet. Nephrol Dial Transplant. 1997;12:924–932. doi: 10.1093/ndt/12.5.924. [DOI] [PubMed] [Google Scholar]
- Allegra V, Mengozzi G, Martimbianco L, Vasile A. Early and late effects of erythropoietin on glucose metabolism in maintenance hemodialysis patients. Am J Nephrol. 1996;16:304–308. doi: 10.1159/000169014. [DOI] [PubMed] [Google Scholar]
- Audran M, Gareau R, Matecki S, Durand F, Chenard C, Sicart M-T, Marion B, Bressolle F. Effects of erythropoietin administration in training athletes and possible indirect detection in doping control. Med Sci Sports Exerc. 1999;31:639–645. doi: 10.1097/00005768-199905000-00003. [DOI] [PubMed] [Google Scholar]
- Berglund B, Ekblom B. Effect of recombinant human erythropoietin treatment on blood pressure and some haematological parameters in healthy men. J Intern Med. 1991;229:125–130. doi: 10.1111/j.1365-2796.1991.tb00319.x. [DOI] [PubMed] [Google Scholar]
- Birkeland KI, Stray-Gundersen JIM, Hemmersbach P, Hallen J, Haug E, Bahr R. Effect of rhEPO administration on serum levels of sTfR and cycling performance. Med Sci Sports Exerc. 2000;32:1238–1243. doi: 10.1097/00005768-200007000-00009. [DOI] [PubMed] [Google Scholar]
- Bode-Böger SM, Böger RH, Kuhn M, Radermacher J, Frölich JC. Recombinant human erythropoietin enhances vasoconstrictor tone via endothelin-1 and constrictor prostanoids. Kidney Int. 1996;50:1255–1261. doi: 10.1038/ki.1996.435. [DOI] [PubMed] [Google Scholar]
- Borissova AM, Djambazova A, Todorov K, Dakovska L, Tankova T, Kirilov G. Effect of erythropoietin on the metabolic state and peripheral insulin sensitivity in diabetic patients on haemodialysis. Nephrol Dial Transplant. 1993;8:93–. doi: 10.1093/oxfordjournals.ndt.a092282. [DOI] [PubMed] [Google Scholar]
- Briggs J, Skøtt O, Schnermann J. Cellular mechanisms within the juxtaglomerular apparatus. Am J Hypertens. 1990;3:76–80. doi: 10.1093/ajh/3.1.76. [DOI] [PubMed] [Google Scholar]
- Chang C, Chen Y, Modi K, Awar O, Alfrey C, Rice L. Changes of red blood cell surface markers in a blood doping model of neocytolysis. J Investig Med. 2009;57:650–654. doi: 10.2310/JIM.0b013e3181a3914e. [DOI] [PubMed] [Google Scholar]
- Clavell AL, Stingo AJ, Margulies KB, Brandt RR, Burnett JC., Jr Role of endothelin receptor subtypes in the in vivo regulation of renal function. Am J Physiol Renal Physiol. 1995;268:F455–F460. doi: 10.1152/ajprenal.1995.268.3.F455. [DOI] [PubMed] [Google Scholar]
- Connes P, Perrey S, Varray A, Préfaut C, Caillaud C. Faster oxygen uptake kinetics at the onset of submaximal cycling exercise following 4 weeks recombinant human erythropoietin (r-HuEPO) treatment. Pflugers Arch. 2003;447:231–238. doi: 10.1007/s00424-003-1174-0. [DOI] [PubMed] [Google Scholar]
- Dicato M, Plawny L. Erythropoietin in cancer patients: pros and cons. Curr Opin Oncol. 2010;22:307–311. doi: 10.1097/CCO.0b013e32833aa9de. [DOI] [PubMed] [Google Scholar]
- Donnelly S. Why is erythropoietin made in the kidney? The kidney functions as a ‘critmeter’ to regulate the hematocrit. Adv Exp Med Biol. 2003;543:73–87. doi: 10.1007/978-1-4419-8997-0_6. [DOI] [PubMed] [Google Scholar]
- Dunn A, Lo V, Donnelly S. The role of the kidney in blood volume regulation: the kidney as a regulator of the hematocrit. Am J Med Sci. 2007;334:65–71. doi: 10.1097/MAJ.0b013e318095a4ae. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hinze-Selch D, Stawicki S, Aust C, Knolle-Veentjer S, Wilms S, Heinz G, Erdag S, Jahn H, Degner D, Ritzen M, Mohr A, Wagner M, Schneider U, Bohn M, Huber M, Czernik A, Pollmächer T, Maier W, Sirén AL, Klosterkötter J, Falkai P, Rüther E, Aldenhoff JB, Krampe H. Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Mol Psychiatry. 2007;12:206–220. doi: 10.1038/sj.mp.4001907. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, Jahnig P, Herrmann M, Knauth M, Bahr M, Heide W, Wagner A, Schwab S, Reichmann H, Schwendemann G, Dengler R, Kastrup A, Bartels C, for the EPO Stroke Trial Group Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- Freudenthaler SM, Lucht I, Schenk T, Brink M, Gleiter CH. Dose-dependent effect of angiotensin II on human erythropoietin production. Pflugers Arch. 2000;439:838–844. doi: 10.1007/s004249900238. [DOI] [PubMed] [Google Scholar]
- Gossmann J, Burkhardt R, Harder S, Lenz T, Sedlmeyer A, Klinkhardt U, Geiger H, Scheuermann E-H. Angiotensin II infusion increases plasma erythropoietin levels via an angiotensin II type 1 receptor-dependent pathway. Kidney Int. 2001;60:83–86. doi: 10.1046/j.1523-1755.2001.00773.x. [DOI] [PubMed] [Google Scholar]
- Heidenreich S, Rahn K, Zidek W. Direct effects of recombinant human erythropoietin on renal resistance vessels. Kidney Int. 1991;39:259–265. doi: 10.1038/ki.1991.31. [DOI] [PubMed] [Google Scholar]
- Hojman P, Brolin C, Gissel H, Brandt C, Zerahn B, Pedersen BK, Gehl J. Erythropoietin over-expression protects against diet-induced obesity in mice through increased fat oxidation in muscles. PLoS ONE. 2009;4:e5894. doi: 10.1371/journal.pone.0005894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein-Rathlou NH. A closed-loop analysis of the tubuloglomerular feedback mechanism. Am J Physiol Renal Physiol. 1991;261:F880–F889. doi: 10.1152/ajprenal.1991.261.5.F880. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007;78:183–205. doi: 10.1111/j.1600-0609.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- Juel C, Thomsen J, Rentsch R, Lundby C. Effects of prolonged recombinant human erythropoietin administration on muscle membrane transport systems and metabolic marker enzymes. Eur J Appl Physiol. 2007;102:41–44. doi: 10.1007/s00421-007-0567-8. [DOI] [PubMed] [Google Scholar]
- Kokot F, Wiecek A, Mesjasz J, Adamczak M, Spiechowicz U. Influence of long-term recombinant human erythropoietin (rHuEpo) therapy on plasma leptin and neuropeptide Y concentration in haemodialysed uraemic patients. Nephrol Dial Transplant. 1998;13:1200–1205. doi: 10.1093/ndt/13.5.1200. [DOI] [PubMed] [Google Scholar]
- Krapf R, Hulter HN. Arterial hypertension induced by erythropoietin and erythropoiesis-stimulating agents (ESA) Clin J Am Soc Nephrol. 2009;4:470–480. doi: 10.2215/CJN.05040908. [DOI] [PubMed] [Google Scholar]
- Lasne F, Martin L, Crepin N, de Ceaurriz J. Detection of isoelectric profiles of erythropoietin in urine: differentiation of natural and administered recombinant hormones. Anal Biochem. 2002;311:119–126. doi: 10.1016/s0003-2697(02)00407-4. [DOI] [PubMed] [Google Scholar]
- Lundby C, Achman-Andersen NJ, Thomsen JJ, Norgaard AM, Robach P. Testing for recombinant human erythropoietin in urine: problems associated with current anti-doping testing. J Appl Physiol. 2008a;105:417–419. doi: 10.1152/japplphysiol.90529.2008. [DOI] [PubMed] [Google Scholar]
- Lundby C, Damsgaard R. Exercise performance in hypoxia after novel erythropoiesis stimulating protein treatment. Scand J Med Sci Sports. 2006;16:35–40. doi: 10.1111/j.1600-0838.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- Lundby C, Hellsten Y, Jensen MBF, Munch AS, Pilegaard H. Erythropoietin receptor in human skeletal muscle and the effects of acute and long-term injections with recombinant human erythropoietin on the skeletal muscle. J Appl Physiol. 2008b;104:1154–1160. doi: 10.1152/japplphysiol.01211.2007. [DOI] [PubMed] [Google Scholar]
- Lundby C, Robach P, Boushel R, Thomsen JJ, Rasmussen P, Koskolou M, Calbet JAL. Does recombinant human Epo increase exercise capacity by means other than augmenting oxygen transport? J Appl Physiol. 2008c;105:581–587. doi: 10.1152/japplphysiol.90484.2008. [DOI] [PubMed] [Google Scholar]
- Lundby C, Thomsen JJ, Boushel R, Koskolou M, Warberg J, Calbet JAL, Robach P. Erythropoietin treatment elevates haemoglobin concentration by increasing red cell volume and depressing plasma volume. J Physiol. 2007;578:309–314. doi: 10.1113/jphysiol.2006.122689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak K, Inkster B, O'Sullivan U, Selvaraj S, Goodwin GM, Harmer CJ. Differential effects of erythropoietin on neural and cognitive measures of executive function 3 and 7 days post-administration. Exp Brain Res. 2008;184:313–321. doi: 10.1007/s00221-007-1102-1. [DOI] [PubMed] [Google Scholar]
- Miskowiak KW, Favaron E, Hafizi S, Inkster B, Goodwin GM, Cowen PJ, Harmer CJ. Effects of erythropoietin on emotional processing biases in patients with major depression: an exploratory fMRI study. Psychopharmacology (Berl) 2009;207:133–142. doi: 10.1007/s00213-009-1641-1. [DOI] [PubMed] [Google Scholar]
- Ninot G, Connes P, Caillaud C. Effects of recombinant human erythropoietin injections on physical self in endurance athletes. J Sports Sci. 2006;24:383–391. doi: 10.1080/02640410500131340. [DOI] [PubMed] [Google Scholar]
- Olsen NV, Aachmann-Andersen NJ, Oturai P, Andersen TM, Rasmussen AB, Hulston C, Holstein-Rathlou N-H, Robach P, Lundby C. Recombinant human erythropoietin in humans down-regulates proximal renal tubular reabsorption and causes a fall in glomerular filtration rate. J Physiol. 2010;589:1273–1281. doi: 10.1113/jphysiol.2010.194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisotto R, Gore C, Emslie K, Ashenden M, Brugnara C, Howe C, Martin D, Trout G, Hahn A. A novel method utilising markers of altered erythropoiesis for the detection of recombinant human erythropoietin abuse in athletes. Haematologica. 2000;85:564–572. [PubMed] [Google Scholar]
- Pfeffer MA, Burdmann EA, Chen C-Y, Cooper ME, de Zeeuw D, Eckardt K-U, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJV, Parfrey P, Parving H-H, Remuzzi G, Singh AK, Solomon SD, Toto R, the TREAT Investigators A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369:381–388. doi: 10.1016/S0140-6736(07)60194-9. [DOI] [PubMed] [Google Scholar]
- Pratt MC, Lewis-Barned NJ, Walker RJ, Bailey RR, Shand Bl, Livesey J. Effect of angiotensin converting enzyme inhibitors on erythropoietin concentrations in healthy volunteers. Br J Clin Pharmacol. 1992;34:363–365. doi: 10.1111/j.1365-2125.1992.tb05644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen P, Foged EM, Krogh-Madsen R, Nielsen J, Nielsen TR, Olsen NV, Petersen NC, Sorensen TA, Secher NH, Lundby C. Effects of erythropoietin administration on cerebral metabolism and exercise capacity in men. J Appl Physiol. 2010;109:476–483. doi: 10.1152/japplphysiol.00234.2010. [DOI] [PubMed] [Google Scholar]
- Resch BE, Gaspar R, Sonkodi S, Falkay G. Vasoactive effects of erythropoietin on human placental blood vessels in vitro. Am J Obstet Gynecol. 2003;188:993–996. doi: 10.1067/mob.2003.211. [DOI] [PubMed] [Google Scholar]
- Robach P, Cairo G, Gelfi C, Bernuzzi F, Pilegaard H, Vigano A, Santambrogio P, Cerretelli P, Calbet JAL, Moutereau S, Lundby C. Strong iron demand during hypoxia-induced erythropoiesis is associated with down-regulation of iron-related proteins and myoglobin in human skeletal muscle. Blood. 2007;109:4724–4731. doi: 10.1182/blood-2006-08-040006. [DOI] [PubMed] [Google Scholar]
- Robach P, Calbet JAL, Thomsen JJ, Boushel R, Mollard P, Rasmussen P, Lundby C. The ergogenic effect of recombinant human erythropoietin on VO2max depends on the severity of arterial hypoxemia. PLoS ONE. 2008;3:e2996. doi: 10.1371/journal.pone.0002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robach P, Recalcati S, Girelli D, Gelfi C, Aachmann-Andersen NJ, Thomsen JJ, Norgaard AM, Alberghini A, Campostrini N, Castagna A, Vigano A, Santambrogio P, Kempf T, Wollert KC, Moutereau S, Lundby C, Cairo G. Alterations of systemic and muscle iron metabolism in human subjects treated with low-dose recombinant erythropoietin. Blood. 2009;113:6707–6715. doi: 10.1182/blood-2008-09-178095. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Leibel RL. The role of leptin in human physiology. N Engl J Med. 1999;341:913–915. doi: 10.1056/NEJM199909163411211. [DOI] [PubMed] [Google Scholar]
- Rundqvist H, Rullman E, Sundberg CJ, Fischer H, Eisleitner K, Stahlberg M, Sundblad P, Jansson E, Gustafsson T. Activation of the erythropoietin receptor in human skeletal muscle. Eur J Endocrinol. 2009;161:427–434. doi: 10.1530/EJE-09-0342. [DOI] [PubMed] [Google Scholar]
- Russell G, Gore C, Ashenden M, Parisotto R, Hahn A. Effects of prolonged low doses of recombinant human erythropoietin during submaximal and maximal exercise. Eur J Appl Physiol. 2002;86:442–449. doi: 10.1007/s00421-001-0560-6. [DOI] [PubMed] [Google Scholar]
- Scalera F, Kielstein JT, Martens-Lobenhoffer J, Postel SC, Tager M, Bode-Boger SM. erythropoietin increases asymmetric dimethylarginine in endothelial cells: role of dimethylarginine dimethylaminohydrolase. J Am Soc Nephrol. 2005;16:892–898. doi: 10.1681/ASN.2004090735. [DOI] [PubMed] [Google Scholar]
- Sinclair AM, Coxon A, McCaffery I, Kaufman S, Paweletz K, Liu L, Busse L, Swift S, Elliott S, Begley CG. Functional erythropoietin receptor is undetectable in endothelial, cardiac, neuronal, and renal cells. Blood. 2010;115:4264–4272. doi: 10.1182/blood-2009-10-248666. [DOI] [PubMed] [Google Scholar]
- Thomsen J, Rentsch R, Robach P, Calbet J, Boushel R, Rasmussen P, Juel C, Lundby C. Prolonged administration of recombinant human erythropoietin increases submaximal performance more than maximal aerobic capacity. Eur J Appl Physiol. 2007;101:481–486. doi: 10.1007/s00421-007-0522-8. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Vaziri ND. Erythropoietin depresses nitric oxide synthase expression by human endothelial cells. Hypertension. 1999;33:894–899. doi: 10.1161/01.hyp.33.3.894. [DOI] [PubMed] [Google Scholar]
- Wilkerson DP, Rittweger J, Berger NJA, Naish PF, Jones AM. Influence of recombinant human erythropoietin treatment on pulmonary O2 uptake kinetics during exercise in humans. J Physiol. 2005;568:639–652. doi: 10.1113/jphysiol.2005.089920. [DOI] [PMC free article] [PubMed] [Google Scholar]


