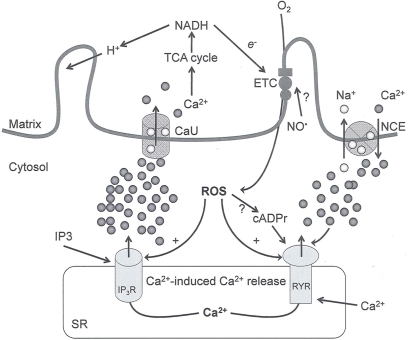
Figure 2.
Interaction between mitochondria and SR Ca2+-release channels. This structural arrangement allows mitochondria and SR to be connected by tether proteins (not shown), which allows regulation of matrix Ca2+ that may modulate mitochondrial activity. Mitochondria take up Ca2+ ions entering the cytosol from internal stores (SR) of the local “microdomain” via signaling molecules that include Ca2+ and ROS. After cell stimulation, inositol 1,4,5-trisphosphate (IP3) and cADP ribose (cADPr) release calcium from SR through their respective receptors [IP3R and ryanodine receptors (RyR)]. Local increases in cytosolic Ca2+ concentrations are buffered by mitochondrial uptake through the calcium uniporter (CaU) and possible alternative routes, such as the putative Ca2+/H+ exchanger [the so-called Na+ independent Ca2+ exchanger (NICE, or CHE); not shown]. Intra-mitochondrial Ca2+ may activate synthesis of reduced substrates (NADH) by metabolic pathways and accelerate the electron transport chain (ETC); a resulting increase in ROS production may in turn facilitate Ca2+ release by sensitization of IP3R and RyR. The increase in respiration is associated with enhanced proton pumping and subsequently increase state 3 respiration with perhaps increase CHE activity so that more Ca2+ is taken up from the localized “microdomain.” Other putative mechanisms that increase ROS generation are modifications of ETC complexes by mCa2+ and inhibition of the ETC by nitric oxide (). The can be increased by Ca2+-induced synthase. Mitochondrial can also support Ca2+ release by efflux via Na+/Ca2+ exchange (NCE). The delicate balance in the regulation of Ca2+ in the “microdomain” is crucial in the pathology of mitochondria-related cardiovascular complications. Reproduced and modified by permission from Camello-Almaraz et al. (2006).