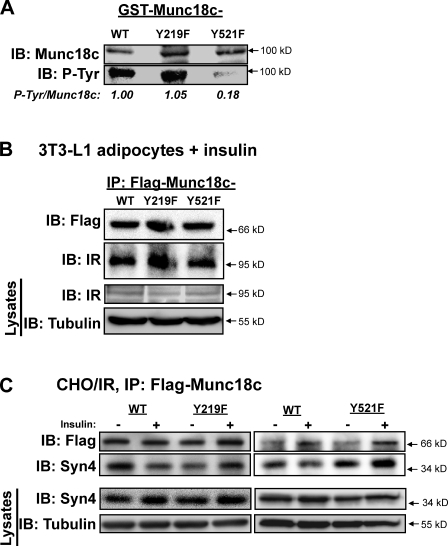
Figure 8.
IR binding and phosphorylation of Munc18c at Tyr219 and Tyr521 and the requirement for each to mediate Munc18c dissociation from syntaxin 4. (A) Recombinantly expressed and purified GST-Munc18c-WT, GST-Munc18c-Y219F, and GST-Munc18c-Y521F proteins linked to glutathione–Sepharose beads were incubated in the presence of nonradiolabeled ATP and the active β subunit of the IR for 30 min. Reactions were stopped by the addition of Laemmli sample buffer, and GST fusion proteins were pelleted and stringently washed for resolution on 10% SDS-PAGE for the detection of phosphorylation using the antityrosine phosphorylation antibody 4G10. Expression and capture of GST fusion proteins were validated by immunoblotting (IB) for Munc18c, and data from two to three independent experiments using different protein batches were quantified as tyrosine-phosphorylated (P-Tyr) Munc18c/GST-Munc18c, normalized in each to WT = 1. (B) Electroporated adipocytes expressing Flag-Munc18c-WT, -Y219F, or -Y521F mutants were incubated in serum-free medium for 2 h and stimulated with insulin for 5 min for subsequent detergent lysis and utilization in anti-Flag immunoprecipitation (IP) reactions to detect an association with endogenous IR by immunoblotting. Equivalent IR abundance in corresponding starting lysates was confirmed on a separate gel, with tubulin as a loading control (Lysates). (C) CHO/IR cells coelectroporated to express Flag-Munc18c-WT, -Y219F, or -Y521F mutants with full-length syntaxin 4 (Syn4) were incubated in serum-free medium for 2 h and left unstimulated or were insulin stimulated for 30 min for subsequent detergent lysis and utilization in anti-Flag immunoprecipitation reactions to detect dissociation from endogenous syntaxin 4 by immunoblotting as previously described (Thurmond et al., 1998). Reactions with Y219F or Y521F, each with a paired WT-positive control, were resolved on two separate gels. Equivalent syntaxin 4 expression in corresponding starting lysates was confirmed on a separate gel, with tubulin as a loading control (Lysates). (B and C) Data are representative of at least three independent experiments using multiple DNA batches.