Abstract
Smooth muscle, striated muscle, their central and peripheral innervations and control, and mucosal coaptation contribute to maintenance of continence. We used manual leak point pressure (mLPP) testing and electrical stimulation LPP (eLPP) testing in female rats to quantify the contribution of these factors to urethral resistance, a measure of continence. Abdominal muscles were electrically stimulated to induce leakage for eLPP. A Crede maneuver was applied for mLPP. These were repeated after complete T8 spinal cord injury (SCI) and/or bilateral pudendal nerve transection (PNT). After euthanasia, mLPP was repeated. MLPP was not significantly affected by opening the abdomen, suggesting that intra-abdominal pressure transmission contributes little to continence during slow pressure changes. ELPP was significantly higher than mLPP in intact rats, after PNT, and after SCI+PNT, suggesting that abdominal pressure transmission contributes to continence during rapid increases in intra-abdominal pressure. MLPP decreased significantly after PNT, indicating that urethral striated muscles contribute significantly to continence. ELPP decreased significantly after PNT with and without SCI, suggesting that supraspinal control significantly affects continence during rapid pressure changes, but not during slow pressure changes. MLPP after euthanasia was significantly decreased compared to mLPP after SCI+PNT, suggesting that urethral mucosal seal coaptation and tissue elasticity also contribute to continence. The urethra is a complex organ that maintains continence via a highly organized and hierarchical system involving both the central and peripheral nervous systems.
Keywords: neurophysiology, urinary incontinence, regulatory, guarding reflex, rat, urodynamics
1. Introduction
Except for drainage of urine during voiding, urethral function is primarily to maintain closure pressure above that of the bladder to maintain continence (de Groat et al., 2001). Insufficient urethral resistance to leakage and/or urethral support can result in stress urinary incontinence (SUI) which occurs when vesical or bladder pressure exceeds urethral resistance as a result of increased intra-abdominal pressure in the absence of a bladder contraction (Rovner and Wein 2004). The tube-like urethra is a complex organ that includes the epithelial lining, muscular walls, including longitudinal and circumferential smooth muscles, striated muscle, and a structural support system. Deficiency in any of these elements can result in decreased urethral resistance and urine leakage (Ashton-Miller and DeLancey 2007; Chancellor et al., 2005; Haab et al., 1996).
Urethral resistance depends on integration of anatomic support and neurophysiologic control in this complex system (Ashton-Miller and DeLancey 2007; Haab et al., 1996), including contributing factors such as abdominal pressure transmission (Enhorning 1961), mucosal seal coaptation (Stanton et al., 1978; Zinner et al., 1980), smooth muscle contraction (Andersson and Wein 2004), supraspinal control (Miller et al., 1995; Nakagawa 1980), striated muscle contraction, and pelvic floor support (Chancellor and Yoshimura 2004; Wein 2007). Quantification of the contribution of each of these factors would lead to a better understanding of the physiology of this complex organ. However, the quantitative contribution of each of these factors to urethral resistance has not previously been determined.
The aim of this study was to determine the portion of urethral resistance provided by different contributing physiological or mechanical factors in female rats, including abdominal pressure transmission, mucosal seal coaptation of the urethra, as well as urethral smooth and striated muscle contraction and their central and peripheral innervation. We used the manual leak point pressure (mLPP) test which mimics the Crede maneuver (Cannon and Damaser 2001) and the electrical stimulation LPP (eLPP) test which mimics a sudden increase in abdominal pressure (Kamo and Hashimoto 2007; Widdicombe 1995) in conjunction with acute denervation and other disruptive studies to quantify the contribution of each factor.
2. Results
2.1 ELPP and mLPP in intact rats
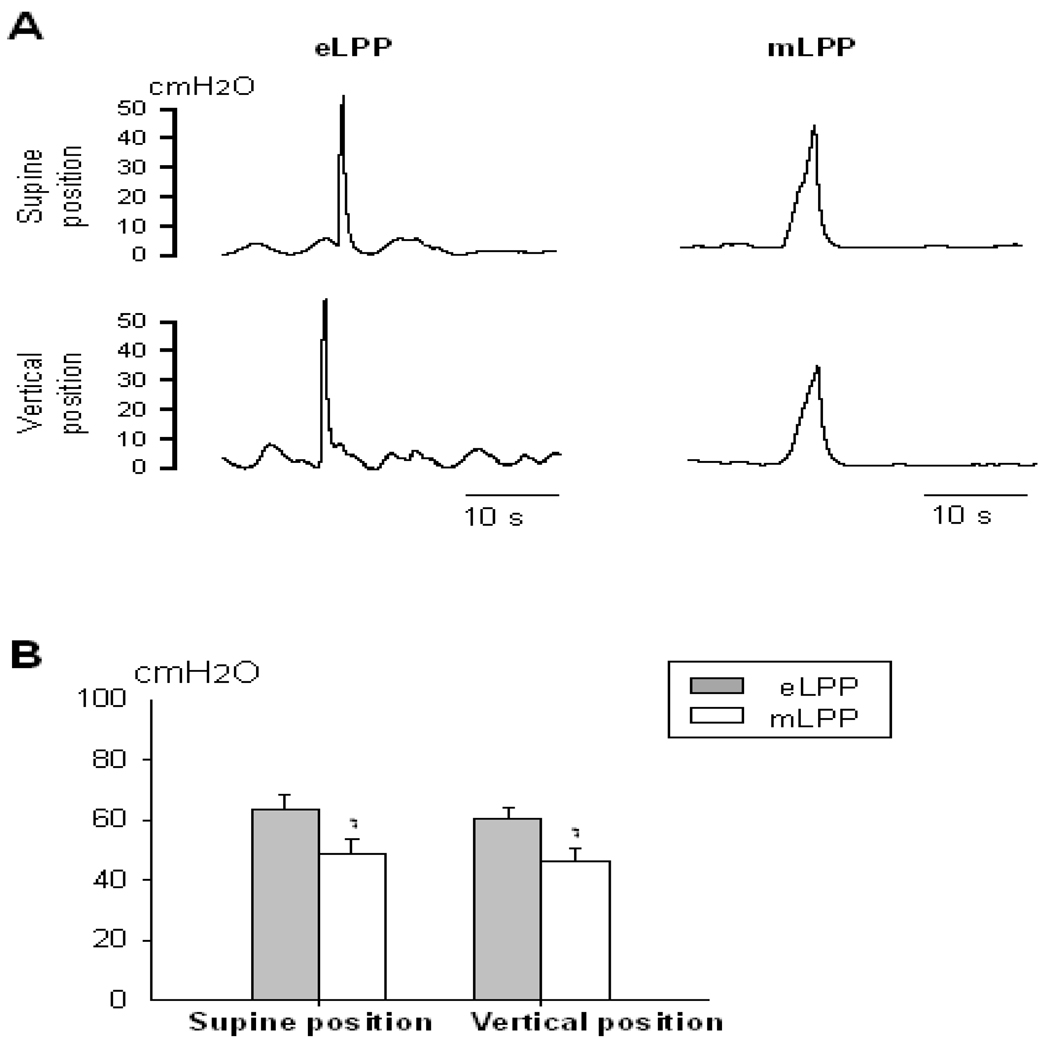
In rats showing leakage during eLPP testing (n=11), eLPP was significantly higher than mLPP in both supine and vertical positions (Fig. 1). However, neither eLPP nor mLPP was significantly affected by the change in position. In rats that showed no leakage with eLPP testing (n=5), mLPP did not significantly decrease after opening the abdominal wall (mLPP with abdominal wall intact: 50.86 ± 7.01 cm H2O; mLPP with abdominal wall open: 45.76 ± 4.28 cm H2O), suggesting that abdominal pressure transmission does not contribute to urethral resistance as measured by mLPP.
Fig. 1.
Electrostimulation leak point pressure (eLPP) and manual leak point pressure (mLPP) in intact rats. A. Examples of vesical pressure recordings during eLPP and mLPP testing in supine and vertical positions. B. Comparisons of eLPP and mLPP in both supine and vertical positions. * indicates a significant difference compared to eLPP in the same position (p<0.01). Data is presented as mean ± standard error of the mean of data from 11 animals.
2.2 Effects of complete spinal cord injury (SCI) followed by bilateral pudendal nerve transection (PNT) (n=6)
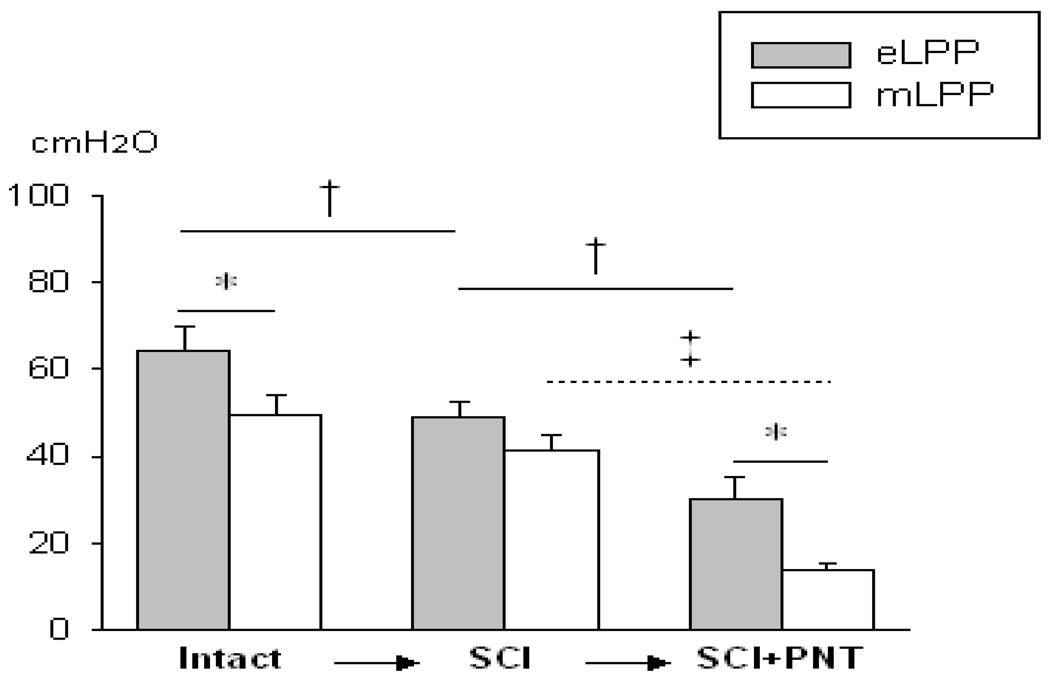
After SCI, consisting of a complete transection at the spinal cord level of T8, eLPP was significantly decreased (Fig. 2), suggesting a significant supraspinal contribution to this simulated cough reflex. After SCI plus PNT, eLPP was further and significantly reduced. No significant decrease in mLPP occurred after SCI, but mLPP decreased significantly after PNT was added (Fig. 2), suggesting that urethral striated muscles contribute to urethral resistance as measured by both eLPP and mLPP. After SCI+PNT, eLPP was significantly higher than mLPP (Fig. 2).
Fig. 2.
Effects of spinal cord injury (SCI) followed by pudendal nerve transection (PNT). Comparisons of electrostimulation leak point pressure (eLPP) and manual leak point pressure (mLPP) in intact rats, after SCI, and after SCI+PNT. * indicates a significant difference between eLPP and comparable mLPP. † indicates that eLPP decreased significantly after SCI and after SCI+PNT. ‡ indicates that mLPP decreased significantly after SCI+PNT. Data is presented as mean ± standard error of the mean of data from 6 animals.
2.3 Effects of PNT followed by SCI (n=5)
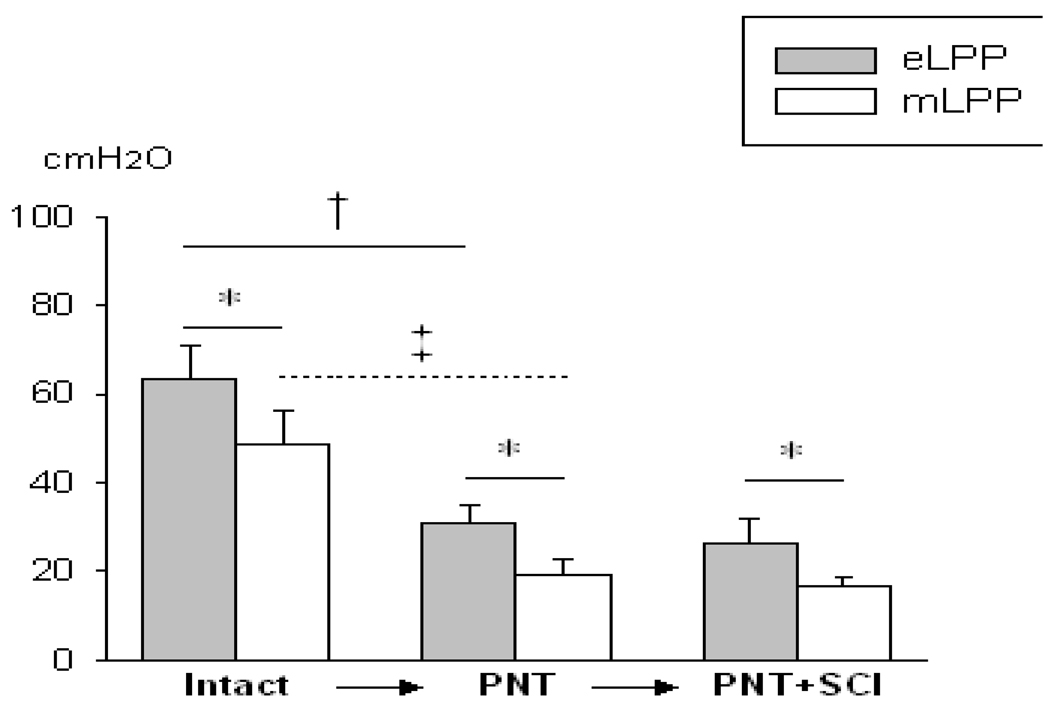
As described above, both eLPP and mLPP demonstrated a significant decrease after PNT (Fig. 3). Furthermore, neither eLPP nor mLPP decreased significantly when SCI was added. MLPP was significantly lower than eLPP in intact animals, after PNT, and after PNT+SCI (Fig. 3), suggesting eLPP recruits additional contributors to urethral resistance compared to mLPP.
Fig. 3.
Effects of pudendal nerve transection (PNT) followed by spinal cord injury (SCI). Comparisons of electrostimulation leak point pressure (eLPP) and manual leak point pressure (mLPP) in intact rats, after PNT, and after PNT+SCI. * indicates a significant difference between eLPP and mLPP. † indicates that eLPP decreased significantly after PNT. ‡ indicates that mLPP decreased significantly after PNT. Data is presented as mean ± standard error of the mean of data from 5 animals.
2.4 mLPP after PNT+SCI and euthanasia (n=11)
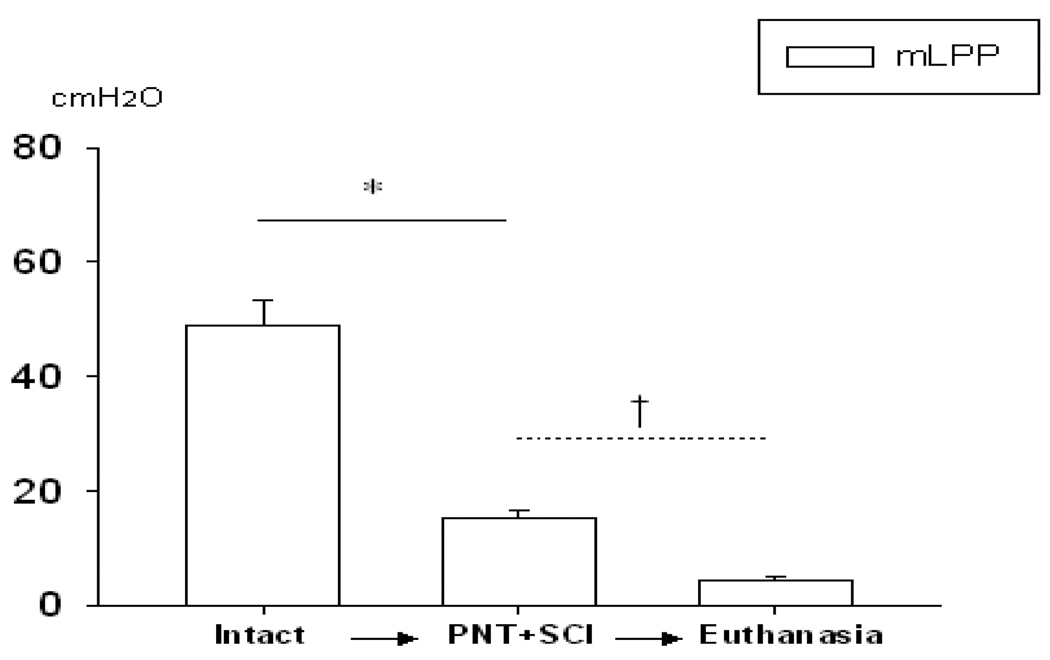
When all 11 animals are considered together (Fig. 2 and 3), mLPP decreased significantly after PNT+SCI compared to mLPP in intact animals. Furthermore, after euthanasia, mLPP was significantly decreased compared to mLPP after PNT+SCI (Fig. 4), suggesting that urethral mucosal coaptation and tissue elasticity, the only contributing factors remaining after euthanasia have a small contribution to urethral resistance.
Fig. 4.
Manual leak point pressure (mLPP) after both pudendal nerve transection (PNT) and spinal cord injury (SCI) followed by euthanasia. Comparisons of mLPP in intact rats, after PNT+SCI, and after euthanasia. * indicates that mLPP decreased significantly after PNT+SCI. † indicates that mLPP decreased significantly after euthanasia. Data is presented as mean ± standard error of the mean of data from 11 animals.
3. Discussion
Along with the pelvic floor support structures, urethral resistance to leakage is generally produced by urethral striated muscle, urethral smooth muscle, their innervations and urethral mucosa. Based on clinical studies, they are generally believed to contribute equally to urethral resistance (Ashton-Miller and DeLancey 2007). Although quadrupedal animals have inherent differences with humans with regard to urinary continence and no animal perfectly represents the clinical situation, animal studies enable testing under controlled conditions and implementation of interventions, such as nerve transection and euthanasia, which are not possible in clinical studies. Urethral resistance in this study did not focus on pelvic floor support structures which likely reflect the greatest differences between species and postures. Therefore, we utilized rats, the animal model most commonly used for physiologic studies of the lower urinary tract, to quantify the portion that each of these factors contribute to urethral resistance. We used LPP, the lowest recorded bladder pressure resulting in urinary leakage, to quantify urethral resistance since it is considered to be the best method to characterize urethral resistance both for clinical and basic science research (Hijaz et al., 2008; Kelly and Krane 2000; Lin et al., 1998). We expect that quantification of these factors will provide a better understanding of the physiology of the mechanism of urethral resistance, and will help differentiate the mechanisms of incontinence. Evaluation of the contribution of different physiologic or biomechanical factors in a patient is critical to selection of appropriate SUI treatment options (Blaivas and Olsson 1988; Rovner and Wein 2004).
Urethral resistance is sometimes tested in a vertical position since clinically most urine leakage occurs when erect (Conway et al., 2005; Takahashi et al., 2006). To assess the contribution of position to urethral resistance in female rats, we tested both methods of LPP in both supine and vertical positions. Neither eLPP nor mLPP was significantly affected by the change in position, suggesting they are similar and repeatable. Although changing posture from a supine to standing position significantly increases urethral pressure at the bladder neck and midurethra in healthy women and women with SUI, maximum urethral closure pressure was unchanged in healthy females while it decreased significantly in women with SUI (Lose 1990; Vereecken 2000). Our results, demonstrating a lack of significant dependence of urethral resistance on posture in female rats, is consistent with this clinical situation, suggesting that changing posture does not significantly affect urethral resistance when pelvic floor structures and support remain intact. Therefore, in our urethral resistance studies in rats, we did not mimic the vertical position of humans.
3.1 Abdominal pressure transmission
Increases in intra-abdominal pressure are generally considered to be transmitted equally to the bladder and the proximal urethra, increasing urethral resistance and preventing urinary leakage (Enhorning 1961). In our study, mLPP was not significantly affected by opening the abdomen, suggesting that abdominal pressure transmission contributes little to urethral resistance during mLPP. In this method, since the external pressure is applied slowly to the abdominal area directly over the bladder, it is not transmitted to the urethra.
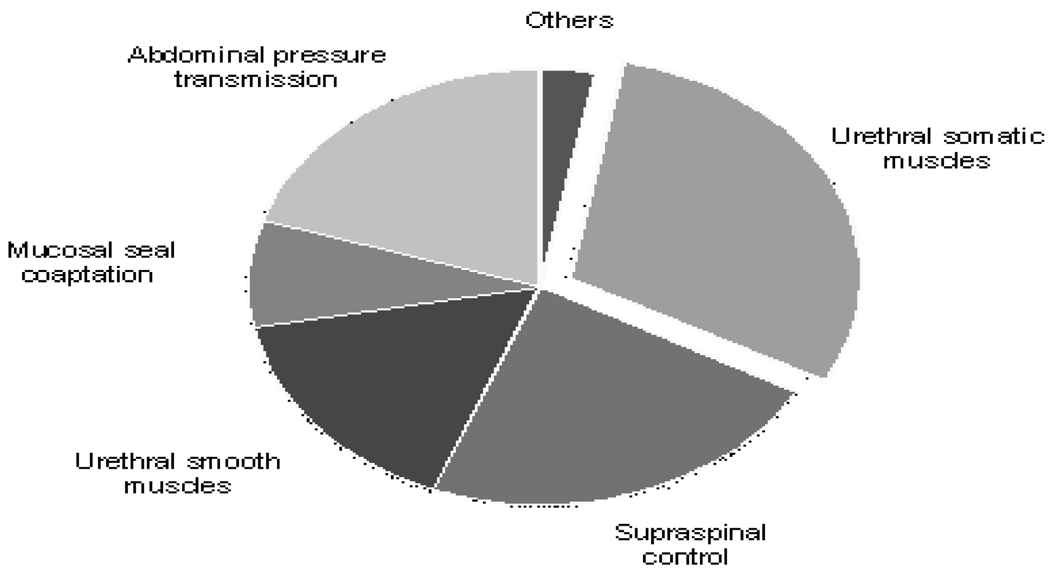
In contrast, during eLPP testing, the sudden abdominal pressure increase from contraction of abdominal muscles was transmitted to the bladder and caused a urethral pressure increase but no bladder contraction. As a result, eLPP was significantly higher than mLPP in intact animals, after PNT, and after SCI+PNT. Compared to mean eLPP in intact rats, mean mLPP was decreased 21% in intact rats, 19% after PNT, and 20% after SCI +PNT. These consistent ratios suggest that abdominal pressure transmission contributes approximately 20% to urethral resistance during a simulated cough reflex (Fig. 5).
Fig. 5.
Schematic of the different contributing factors to urethral resistance showing quantitative contributions.
Abdominal pressure transmission is therefore one of the important factors contributing to urethral resistance during a sudden abdominal pressure increase. If individuals present with no difference in LPP between a sudden increase in abdominal pressure, such as during a cough, and a slower Crede maneuver, the deficient factor may mainly involve abdominal pressure transmission. Incorporation of the deficiencies of urethral support and unbalanced pressure transmission may result from urethral hypermobility, which could also impair other factors contributing to urethral resistance, and may be enhanced by a surgical procedure, such as fascial sling or Burch colposuspension (Albo et al., 2007).
The eLPP test has been utilized previously in rats to induce urinary leakage (Kamo and Hashimoto 2007). Electrical stimulation causes the abdominal muscles to contract suddenly, which produces a physiological cough reflex (Widdicombe 1995). In this study, we primarily consider the effect of stimulation on abdominal pressure, although potential confounding variables may be anticipated. Electrical stimulation may also directly contribute afferent signals to the central nervous system and thus impact reflexes and the bladder indirectly (Delitto and Snyder-Mackler 1990). However, bladder contractions rarely resulted from eLPP testing. We therefore believe the major end result of eLPP testing is to increase abdominal pressure with greater abdominal pressure transmission to the bladder than mLPP.
3.2 Mucosal seal coaptation of the urethra
The mucosal layer is surrounded by the other layers of the urethra and can create a functional seal, facilitating urinary continence (Zinner et al., 1980). When a catheter is placed transurethrally, the mucosa and elasticity of the urethra contribute a sealing effect to urethral resistance by preventing leakage around the catheter (Zinner et al., 1980). Estrogen may also help proliferation and maturation of the urethral epithelium (Willhite and O'Connell 2001). Mucosal seal coaptation of the urethra should be considered to play a fundamental role in urethral resistance, especially in the absence of active urethral closure by striated and smooth muscles. Frozen or drainpipe urethra after trauma or surgery pulls open the urethra with a loss of elasticity and reduced urethral sealing effect (Stanton et al., 1978). The mucosa can also be damaged during pelvic radiation therapy and may participate in the efficacy of injection therapy for urinary incontinence (Appell and Davila 2007; Wein 2007).
In this study, during mLPP testing after pentobarbital euthanasia there was no leakage until a certain pressure was reached. In this case, the factors contributing to urethral resistance can be eliminated except the seal effect of the mucosa and passive elasticity of the urethra. Mean mLPP after euthanasia was approximately 8% of mean eLPP in intact animals, indicating that mucosal sealing and passive elasticity contribute to urethral resistance but do not dominate (Fig. 5). As a result, we expect that an intervention to increase mucosal coaptation in the urethra will only have a significant effect on urethral resistance if urethral muscles do not contract properly. On the contrary, the other contributing factors could be seriously impaired in frozen urethra because of the fundamental role of mucosal seal coaptation during urinary continence.
3.3 Urethral smooth muscles
Smooth muscles, both in the bladder neck and urethra, are involved in opening and closing the urethra. Active closure of the bladder neck depends on α-adrenergic fibers from the hypogastric plexus and α1-adrenoceptors on smooth muscles (Andersson and Wein 2004). Urethral smooth muscles are also regulated by parasympathetic nerves via nitric oxide which exert a relaxation effect (Bennett BC etal., 1995; Andersson and Wein 2004). In this study, we estimate smooth muscle contribution indirectly. After euthanasia, mLPP was significantly decreased compared to after SCI+PNT. This drop in mLPP likely resulted from a loss of smooth muscle activity, since it is the dominant factor contributing to urethral resistance after PNT+SCI. The mean decrease of mLPP from PNT+SCI to euthanasia was approximately 16% of mean eLPP in intact animals, confirming the results of previous experiments using a transection procedure or pharmacological block (Chancellor et al., 2005; de Groat and Yoshimura 2001).
Urethral smooth muscle closure may be evaluated pharmacologically, however the contribution of the different neurotransmitters and their receptors to urethral closure is not fully understood (de Groat and Yoshimura 2001). In addition, systemic administration would have side effects that may affect urethral closure (Bennett et al., 1995). Since much of our experiment was performed serially on the same animals, we were concerned that pharmacological inhibition could affect these serial procedures. Therefore, we did not use a pharmacological block to determine the contribution of smooth muscle to urethral resistance. Moreover, we did not perform hypogastric nerve transection to determine the contribution of smooth muscle even though preganglionic neurons of the hypogastric plexus provide the primary innervation of urethral smooth muscle (de Groat and Yoshimura 2001). This choice was made because such a transection may not fully eliminate the contribution of smooth muscle to urethral resistance since sacral splanchnic nerves and pelvic splanchnic nerves join the inferior hypogastric plexus below the hypogastric nerve (Drake et al., 2005) and nitric oxide can also systemically affect smooth muscle activation (Bennett et al., 1995).
3.4 Supraspinal control
Supraspinal control during bladder filling and urine storage consists of modulation of spinal continence reflexes involving both afferent and efferent innervation of the pelvic and pudendal nerves, pudendal motor neurons to the urethral striated musculature, and their interneurons (Wein 2007). This complex physiological system results in supraspinal regulation of urethral resistance via activation of the motoneurons of urethral striated muscles via both voluntary and involuntary descending pathways (Birder et al., 2010; Miller et al., 1995; Nakagawa 1980). There may also be supraspinal regulation of sympathetic preganglionic neurons at the thoracolumbar region of the spinal cord (Wein 2007).
There was not a significant decrease in mLPP either after SCI in intact rats or after SCI in PNT rats, suggesting that supraspinal control has little contribution to urethral resistance as measured by mLPP. In contrast, after SCI, eLPP decreased significantly by 23% but only decreased further by 7% (not a statistically significant change) after adding PNT (Fig. 5). This suggests that the supraspinal cord participates in urethral resistance as measured by eLPP since it simulates a cough reflex and that this modulation is primarily via pudendal motor neurons. Onuf’s nucleus, which contains the motoneurons of the pudendal nerve, receives descending innervation from the paraventricular hypothalamus and the L region of the pons (Holstege et al., 1986; Holstege and Tan 1987). Their exact function remains unclear but they play an important role in maintain continence during a cough reflex.
3.5 Urethral striated muscles
Urethral striated muscles, primarily in the EUS, are innervated by myelinated somatic nerve fibers from the S2–S4 level of the spinal cord that travel in the pudendal nerve, and actively contribute to urethral resistance by reflex activity both during urine storage and during intra-abdominal pressure increases (Blaivas 1982; Chancellor and Yoshimura 2004). Bilateral transection of the pudendal nerve has been shown to significantly reduce EUS activity and urethral resistance as measured by mLPP (Jiang et al., 2009). The segmental reflex referred to as the guarding or continence reflex has primary importance for urinary continence (Chancellor et al., 2005; Karicheti et al., 2010; Leng and Chancellor 2005). The afferent fibers contributing to this reflex are in the pelvic nerve, and can also include pudendal afferent fibers if urine is entering the urethra (Jiang et al., 2009; Kamo et al., 2004).
Both eLPP and mLPP decreased significantly after PNT by 51% and 47% of mean eLPP in intact animals, respectively. When SCI was added, eLPP and mLPP demonstrated a further drop by 30% and 43% of mean eLPP in intact animals, respectively. Thus, the contribution of urethral striated muscles to urethral resistance is 30% as measured by eLPP and over 40% as measured by mLPP (Fig. 5), suggesting that urethral striated muscles provide the largest single contribution to urethral resistance. This confirms previous studies in both rats (Jiang et al., 2009; Kamo and Hashimoto 2007) and humans (DeLancey et al., 2008).
In summary, the major contributing factors to urethral resistance in female rats include abdominal pressure transmission, mucosal seal coaptation of the urethra, urethral smooth muscles, supraspinal control, and urethral striated muscles. Urethral striated muscles contribute 30–40% to urethral resistance and represent the greatest single contributing factor. A sudden increase in abdominal pressure recruits more factors to urethral resistance than a slower Crede maneuver, including abdominal pressure transmission and supraspinal control. It may be possible to differentiate deficient factors in individuals with SUI by utilizing different measurement methods, which may help direct and specify the course of treatment.
4. Experimental Procedure
The experimental protocols were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic. Age-matched female virgin Sprague-Dawley rats (Harlan, Inc.) weighing 250–300 g underwent bladder filling with saline (5 ml/hr) under urethane anesthesia (1.2 g/kg; i.p.) via a transurethral polyethylene catheter (PE50) connected to both a pressure transducer (model P122; Astro-Med, Inc., West Warwick, RI) and a syringe pump (model 200; KD Scientific Inc., Holliston, MA) to record vesical pressure (DASH 8X; Astro-Med, Inc.) and fill the bladder, respectively. Air pressure was calibrated as zero at the level of the bladder.
4.1 Spinal Cord Injury (SCI) and Pudendal Nerve Transection (PNT)
To create SCI, the spinal cord was exposed and a laminectomy approximately 1–2 vertebra long was made at the T8 level through which the spinal cord was completely transected. The erector spinae muscles and skin were closed separately with 4-0 silk sutures after the transection. To create PNT, the pudendal nerve was transected bilaterally in the ischiorectal fossa proximal to where it innervates the EUS via a gluteal-dorsal approach under microscopy, as we have done previously (Kerns et al., 2000).
4.2 Leak Point Pressure Testing
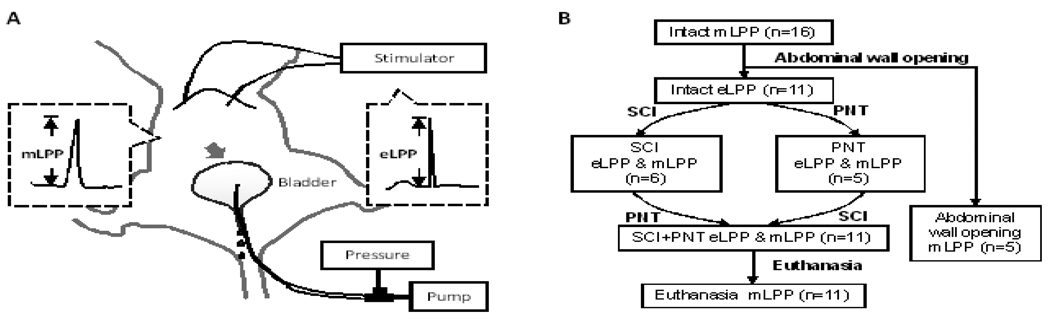
Two 30-gage platinum subdermal electrical stimulation needles (Model F-E2M-48, Grass Inc., West Warwick, RI) were inserted bilaterally into the superior abdominal muscles under the costal margin (Fig. 6A). For eLPP testing (Kamo and Hashimoto 2007), the abdominal muscles were stimulated (50Hz, 0.5ms pulse, 1s duration; Model S88, Grass Inc.) with gradually increasing intensity (10 mA–30 mA) to induce urinary leakage at approximately half bladder capacity (~ 0.3 ml). The stimulation causes the abdominal muscles to contract, which simulates a physiological cough reflex (Widdicombe 1995). The increase in vesical pressure from baseline that caused leakage in the absence of a bladder contraction was defined as eLPP.
Fig. 6.
A. Schematic of the experimental setup for electrostimulation leak point pressure (eLPP) testing and manual leak point pressure (mLPP) testing in female rat. Gray arrow indicates location of external pressure increase in mLPP testing. B. Diagram of experimental design and procedures. SCI, complete spinal cord injury; PNT, bilateral pudendal nerve transection.
For manual LPP testing, vesical pressure was slowly increased by gentle pressure using 2 fingers placed on the closed abdomen directly over the bladder at approximately half bladder capacity as we have done previously (Cannon and Damaser 2001). The external pressure was quickly withdrawn as soon as a leak was observed. The increase in vesical pressure causing leakage of saline in the absence of a bladder contraction was defined as mLPP.
The mLPP test was followed by the eLPP test in each intact rat in both supine and vertical positions. For the latter position, the panel on which the rat was fixed was placed vertically, keeping the pressure transducer at the level of the bladder. Maximum electrical stimulation was determined by gradually increasing the intensity (10 mA–30 mA) until saline leaked from the urethral meatus or the stimulation amplitude reached a maximum of 30mA. Leakage could be induced with eLPP in 11 rats. These animals underwent either complete T8 spinal cord injury (SCI) followed by bilateral pudendal nerve transection (PNT; n=6) or PNT followed by SCI (n=5). Both eLPP and mLPP were repeated after SCI, after PNT, and after both SCI and PNT (Fig. 6B). MLPP was repeated after euthanasia by pentobarbital overdose (200 mg /kg; i.p.).
The 5 rats who did not leak during eLPP testing underwent repeat mLPP testing after a midline abdominal incision to expose the bladder. In this case, an external pressure was applied directly to the bladder via a cotton applicator. Euthanasia followed by repeat mLPP testing was performed as above at the end of the experiments.
LPP in all cases was calculated as baseline pressure subtracted from peak pressure. Each LPP test was repeated 3 times and a mean value for each rat was calculated and used to calculate a mean and standard error for each group. If an active bladder pressure contraction or void was induced by either eLPP or mLPP testing, the result was not included and the test was repeated.
4.3 Statistical Analysis
In rats that showed leakage during eLPP testing (n=11), a paired t-test was used to statistically compare results of eLPP and mLPP testing in the same situation. Two way repeat measures analysis of variance (ANOVA) with the Holm-Sidak posthoc test was used to compare mLPP or eLPP in different situations (Sigma Stat 3.5 and Sigma Plot 10; Systat, Inc. Chicago, IL). In rats showing no leakage during eLPP testing (n=5), a paired t-test was used to compare mLPP results before and after opening the abdominal wall. P<0.05 was used to indicate a statistically significant difference in all comparisons. Data is presented as mean ± standard error of the mean of all animals in each group.
Acknowledgements
This work was supported in part by NIH RO1 HD38679-08, the Cleveland Clinic, and the Rehabilitation Research and Development service of the Department of Veterans Affairs. H.-H. Jiang was supported by the AUA Foundation Research Scholars Program and the Society for Urodynamics and Female Urology. These sources of funding had no direct involvement in the collection, analysis and interpretation of the data; in the writing of the manuscript; and in the decision to submit the paper for publication.
Abbreviations
- mLPP
manual leak point pressure
- eLPP
electrical stimulation LPP
- SCI
spinal cord injury
- PNT
pudendal nerve transaction
- SUI
stress urinary incontinence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hai-Hong Jiang, Email: jiangh@ccf.org.
Levilester B. Salcedo, Email: salcedl@ccf.org.
REFERENCES
- Albo ME, Richter HE, Brubaker L, Norton P, Kraus SR, Zimmern PE, Chai TC, Zyczynski H, Diokno AC, Tennstedt S, Nager C, Lloyd LK, FitzGerald M, Lemack GE, Johnson HW, Leng W, Mallett V, Stoddard AM, Menefee S, Varner RE, Kenton K, Moalli P, Sirls L, Dandreo KJ, Kusek JW, Nyberg LM, Steers W. Urinary Incontinence Treatment Network, 2007. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 356:2143–2155. doi: 10.1056/NEJMoa070416. [DOI] [PubMed] [Google Scholar]
- Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: Basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581–631. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- Appell RA, Davila GW. Treatment options for patients with suboptimal response to surgery for stress urinary incontinence. Curr Med Res Opin. 2007;23:285–292. doi: 10.1185/030079906X162845. [DOI] [PubMed] [Google Scholar]
- Ashton-Miller JA, DeLancey JO. Functional anatomy of the female pelvic floor. Ann N Y Acad Sci. 2007;1101:266–296. doi: 10.1196/annals.1389.034. [DOI] [PubMed] [Google Scholar]
- Bennett BC, Kruse MN, Roppolo JR, Flood HD, Fraser M, de Groat WC. Neural control of urethral outlet activity in vivo: role of nitric oxide. J Urol. 1995;153:2004–2009. [PubMed] [Google Scholar]
- Birder L, de Groat W, Mills I, Morrison J, Thor K, Drake M. Neural control of the lower urinary tract: Peripheral and spinal mechanisms. Neurourol Urodyn. 2010;29:128–139. doi: 10.1002/nau.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaivas JG, Olsson CA. Stress incontinence: Classification and surgical approach. J Urol. 1988;139:727–731. doi: 10.1016/s0022-5347(17)42611-5. [DOI] [PubMed] [Google Scholar]
- Blaivas JG. The neurophysiology of micturition: A clinical study of 550 patients. J Urol. 1982;127:958–963. doi: 10.1016/s0022-5347(17)54147-6. [DOI] [PubMed] [Google Scholar]
- Cannon TW, Damaser MS. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci. 2001;69:1193–1202. doi: 10.1016/s0024-3205(01)01182-1. [DOI] [PubMed] [Google Scholar]
- Chancellor MB, Perkin H, Yoshimura N. Recent advances in the neurophysiology of stress urinary incontinence. Scand J Urol Nephrol. 2005;39:21–24. doi: 10.1080/00365590410002474. [DOI] [PubMed] [Google Scholar]
- Chancellor MB, Yoshimura N. Neurophysiology of stress urinary incontinence. Rev Urol. 2004;6 Suppl 3:S19–S28. [PMC free article] [PubMed] [Google Scholar]
- Conway DA, Kamo I, Yoshimura N, Chancellor MB, Cannon TW. Comparison of leak point pressure methods in an animal model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:359–363. doi: 10.1007/s00192-004-1263-4. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Fraser MO, Yoshiyama M, Smerin S, Tai C, Chancellor MB, Yoshimura N, Roppolo JR. Neural control of the urethra. Scand J Urol Nephrol Suppl. 2001;(207):35–43. doi: 10.1080/003655901750174872. discussion 106–25. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Pharmacology of the lower urinary tract. Annu Rev Pharmacol Toxicol. 2001;41:691–721. doi: 10.1146/annurev.pharmtox.41.1.691. [DOI] [PubMed] [Google Scholar]
- DeLancey JO, Trowbridge ER, Miller JM, Morgan DM, Guire K, Fenner DE, Weadock WJ, Ashton-Miller JA. Stress urinary incontinence: Relative importance of urethral support and urethral closure pressure. J Urol. 2008;179:2286–2290. doi: 10.1016/j.juro.2008.01.098. discussion 2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delitto A, Snyder-Mackler L. Two theories of muscle strength augmentation using percutaneous electrical stimulation. Phys Ther. 1990;70:158–164. doi: 10.1093/ptj/70.3.158. [DOI] [PubMed] [Google Scholar]
- Drake RL, Vogl W, Mitchell AWM. Gray's Anatomy for Students. Philadephia: Elsevier; 2005. [Google Scholar]
- Enhorning G. Simultaneous recording of intravesical and intra-urethral pressure. A study on urethral closure in normal and stress incontinent women. Acta Chir Scand Suppl. 1961 Suppl 276:1–68. [PubMed] [Google Scholar]
- Haab F, Zimmern PE, Leach GE. Female stress urinary incontinence due to intrinsic sphincteric deficiency: Recognition and management. J Urol. 1996;156:3–17. [PubMed] [Google Scholar]
- Hijaz A, Daneshgari F, Sievert KD, Damaser MS. Animal models of female stress urinary incontinence. J Urol. 2008;179:2103–2110. doi: 10.1016/j.juro.2008.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G, Tan J. Supraspinal control of motoneurons innervating the striated muscles of the pelvic floor including urethral and anal sphincters in the cat. Brain. 1987;110(Pt 5):1323–1344. doi: 10.1093/brain/110.5.1323. [DOI] [PubMed] [Google Scholar]
- Holstege G, Griffiths D, de Wall H, Dalm E. Anatomical and physiological observations on supraspinal control of bladder and urethral sphincter muscles in the cat. J Comp Neurol. 1986;250:449–461. doi: 10.1002/cne.902500404. [DOI] [PubMed] [Google Scholar]
- Jiang HH, Pan HQ, Gustilo-Ashby MA, Gill B, Glaab J, Zaszczurynski P, Damaser M. Dual simulated childbirth injuries result in slowed recovery of pudendal nerve and urethral function. Neurourol Urodyn. 2009;28:229–235. doi: 10.1002/nau.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamo I, Hashimoto T. Involvement of reflex urethral closure mechanisms in urethral resistance under momentary stress condition induced by electrical stimulation of rat abdomen. Am J Physiol Renal Physiol. 2007;293:F920–F926. doi: 10.1152/ajprenal.00466.2006. [DOI] [PubMed] [Google Scholar]
- Kamo I, Cannon TW, Conway DA, Torimoto K, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder-to-urethral reflexes in urinary continence mechanisms in rats. Am J Physiol Renal Physiol. 2004;287:F434–F441. doi: 10.1152/ajprenal.00038.2004. [DOI] [PubMed] [Google Scholar]
- Karicheti V, Langdale CL, Ukai M, Thor KB. Characterization of a "spinal - urine storage reflex - inhibitory center" (SUSRIC) and its regulation by serotonin 5-HT1A receptors in female cats. Am J Physiol Regul Integr Comp Physiol. 2010 doi: 10.1152/ajpregu.00599.2009. [DOI] [PubMed] [Google Scholar]
- Kelly CE, Krane RJ. Current concepts and controversies in urodynamics. Curr Urol Rep. 2000;1:217–226. doi: 10.1007/s11934-000-0022-4. [DOI] [PubMed] [Google Scholar]
- Kerns JM, Damaser MS, Kane JM, Sakamoto K, Benson JT, Shott S, Brubaker L. Effects of pudendal nerve injury in the female rat. Neurourol Urodyn. 2000;19:53–69. doi: 10.1002/(sici)1520-6777(2000)19:1<53::aid-nau7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Leng WW, Chancellor MB. How sacral nerve stimulation neuromodulation works. Urol Clin North Am. 2005;32:11–18. doi: 10.1016/j.ucl.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Lin AS, Carrier S, Morgan DM, Lue TF. Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology. 1998;52:143–151. doi: 10.1016/s0090-4295(98)00136-8. [DOI] [PubMed] [Google Scholar]
- Lose G. The impact of changes in posture and bladder filling on the mechanical properties of the urethra in healthy and stress incontinent females. Neurourol Urodyn. 1990;9:459–469. [Google Scholar]
- Miller AD, Nonaka S, Siniaia MS, Jakus J. Multifunctional ventral respiratory group: Bulbospinal expiratory neurons play a role in pudendal discharge during vomiting. J Auton Nerv Syst. 1995;54:253–260. doi: 10.1016/0165-1838(95)00018-s. [DOI] [PubMed] [Google Scholar]
- Nakagawa S. Onuf's nucleus of the sacral cord in a south american monkey (saimiri): Its location and bilateral cortical input from area 4. Brain Res. 1980;191:337–344. doi: 10.1016/0006-8993(80)91285-8. [DOI] [PubMed] [Google Scholar]
- Rovner ES, Wein AJ. Treatment options for stress urinary incontinence. Rev Urol. 2004;6 Suppl 3:S29–S47. [PMC free article] [PubMed] [Google Scholar]
- Stanton SL, Cardozo L, Williams JE, Ritchie D, Allan V. Clinical and urodynamic features of failed incontinence surgery in the female. Obstet Gynecol. 1978;51:515–520. doi: 10.1097/00006250-197805000-00001. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Chen Q, Ogushi T, Fujimura T, Kumagai J, Matsumoto S, Hijikata S, Tabata Y, Kitamura T. Periurethral injection of sustained release basic fibroblast growth factor improves sphincteric contractility of the rat urethra denervated by botulinum-a toxin. J Urol. 2006;176:819–823. doi: 10.1016/j.juro.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Vereecken RL. A critical view on the value of urodynamics in non-neurogenic incontinence in women. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:188–195. doi: 10.1007/pl00004025. [DOI] [PubMed] [Google Scholar]
- Wein AJ. Pathophysiology and classification of voiding dysfunction. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 9th ed. Philadelphia: Saunders/Elsevier; 2007. pp. 1973–1985. [Google Scholar]
- Widdicombe JG. Neurophysiology of the cough reflex. Eur Respir J. 1995;8:1193–1202. doi: 10.1183/09031936.95.08071193. [DOI] [PubMed] [Google Scholar]
- Willhite LA, O'Connell MB. Urogenital atrophy: Prevention and treatment. Pharmacotherapy. 2001;21:464–480. doi: 10.1592/phco.21.5.464.34486. [DOI] [PubMed] [Google Scholar]
- Zinner NR, Sterling AM, Ritter RC. Role of inner urethral softness in urinary continence. Urology. 1980;16:115–117. doi: 10.1016/0090-4295(80)90352-0. [DOI] [PubMed] [Google Scholar]