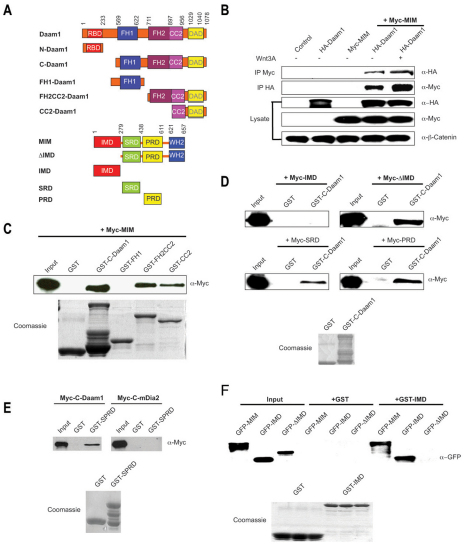
Fig. 1.
MIM is a Daam1-interacting protein. (A) Domain structure of Daam1 and MIM constructs. Numbers indicate amino acid positions. RBD, Rho-binding domain; DAD, diaphanous autoregulatory domain. (B) Full-length Daam1 interacts with full-length MIM and is positively regulated by Wnt3a (3 hours of treatment). HA-Daam1 and Myc-MIM constructs were co-transfected into human HEK293T cells and lysates were immunoprecipitated with the indicated antibodies. (C) MIM interacts with C-Daam1, the FH2-CC2 and CC2 domains, but not the FH1 domain, in GST pull-down assays. (D) The ΔIMD, SRD and PRD, but not the IMD, interact with Daam1. (E) MIM specifically interacts with Daam1 but not with the Formin protein mDia2. (F) MIM self-associates through its IMD. GST pull-down shows that full-length MIM and IMD, but not the ΔIMD, bind to recombinant IMD protein. For pull-downs, Myc-MIM, IMD, ΔIMD, SRD and PRD constructs were transfected into HEK293T cells and lysates were isolated. GST and GST-tagged C-Daam1, FH1, FH2-CC2, CC2, SPRD and IMD recombinant proteins were prepared from transfected E. coli BL21 cells. The integrity of GST-tagged proteins is shown by Coomassie staining.