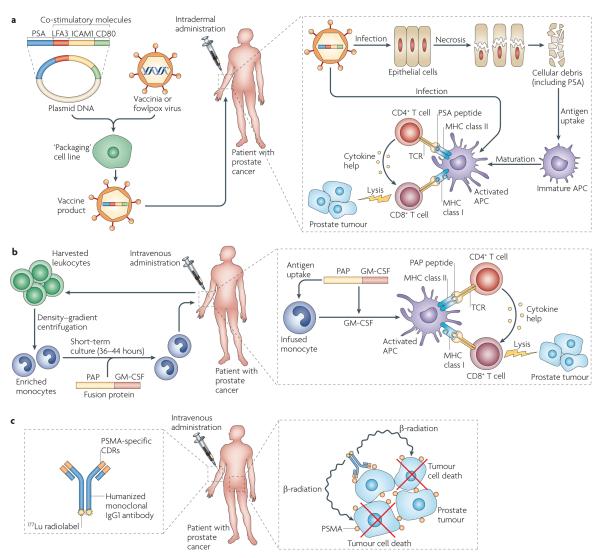
Figure 2. Examples of antigen-specific immunotherapy for prostate cancer.
a ∣ The ProstVac VF ‘vaccine’ consists of a DNA plasmid encoding the target antigen, prostate-specific antigen (PSA), and a series of three co-stimulatory molecules (lymphocyte function-associated antigen 3 (LFA3), CD80 and intercellular adhesion molecule 1 (ICAM1)). The plasmid cassette is incorporated into a poxvirus backbone in a ‘packaging’ cell line, giving a final vaccine product. In this approach, a vaccinia virus-based prime is followed by a fowlpox virus-based boost. The viral vectors are injected intradermally, where they probably infect the patient’s epithelial cells. This in turn leads to epithelial cell death, following which the cellular debris (including the target antigen PSA) is taken up by host antigen-presenting cells (APCs) and presented to host CD4+ and CD8+ T cells. A second potential mechanism for antigen presentation involves direct infection of APCs, including the Langerhans cells in the skin. The incorporation of CD80 into the viral vector facilitates the activation of T cells, through the provision of a co-stimulatory signal for T cell activation. LFA3 and ICAM1 are adhesion molecules; in this context their vector-driven expression on APCs provides additional co-stimulation to facilitate T cell activation. b ∣ Sipuleucel-T immunotherapy is similar to a dendritic cell (DC) vaccine and is based on cells from a patient-derived leukopheresis product. These cells are sent to a central processing facility where monocytes are enriched by density–gradient centrifugation. These monocytes are incubated for 36–44 hours with a specific fusion protein, coupling granulocyte–macrophage colony-stimulating factor (GM-CSF) to the target antigen, in this case prostatic acid phophatase (PAP). In this approach, GM-CSF targets the fusion protein to immature DCs and enhances subsequent DC maturation. Following incubation, the product is sent to the clinic where it is administered intravenously. Once in the patient, the patient’s immature monocytes are thought to mature to fully competent APCs, presenting PAP peptides to the host immune system in a manner that activates CD4+ and CD8+ T cells. c ∣ J591 is an antigen-specific approach using a humanized monoclonal antibody specific for prostate-specific membrane antigen (PSMA). Although early trials used unlabelled antibody, current trials involve 177Lu-labelled J591, a β-ray emitter with a half-life and path-length favourable for radiotherapy and immunotherapy. Here, the antibody specifically targets the radioactive isotope to the target tissue, where tumour cell death is mediated by irradiation. CDR, complementarity-determining region; TCR, T cell receptor.