Table 1.
Analogues and derivatives of oncrasin-1 and their antitumor activities (IC50, μM)
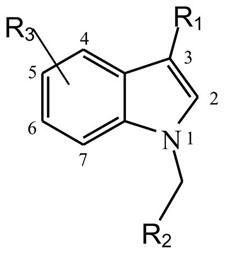 | ||||||
|---|---|---|---|---|---|---|
| Compounds | R1 | R2 | R3 Indole Ring | T29 Cells μM | T29Kt1Cells μM | H460 Cells μM |
| Oncrasin−1 | CHO | 4′-Cl Ph | H | >31.6 | 2.51 | 0.25 |
| 1 | CHO | 2′-F Ph | H | >31.6 | 0.31 | 0.1 |
| 2 | CHO | 3′-F Ph | H | >31.6 | 2.63 | 0.031 |
| 3 | CHO | 3′-Cl Ph | H | >31.6 | 0.37 | 0.037 |
| 4 | CHO | 3′-Br Ph | H | >31.6 | 1.58 | 0.039 |
| 5 | CHO | 4′-Br Ph | H | >31.6 | 0.25 | 0.031 |
| 6 | CHO | 3′-CF3Ph | H | >31.6 | 1.78 | 0.14 |
| 7 | CHO | 4′-CF3Ph | H | >31.6 | >31.6 | 1.58 |
| 8 | CHO | 3′-MePh | H | >31.6 | 6.3 | 0.039 |
| 9 | CHO | 4′-MePh | H | >31.6 | 3.98 | 0.063 |
| 10 | CHO | 3′-NO2Ph | H | >31.6 | 0.50 | 0.039 |
| 11 | CHO | 4′-NO2 Ph | H | >31.6 | 10.0 | 0.50 |
| 12 | CHO | 2′,4′-2Cl Ph | H | >31.6 | 1.58 | 0.1 |
| 13 | CHO | 3′,4′-2Br Ph | H | >31.6 | 0.63 | 0.063 |
| 14 | CHO | Ph | H | >31.6 | 5.0 | 0.045 |
| 15 | CHO | 4′-Cl (CH2)2OPh | H | >31.6 | 4.89 | 0.045 |
| 16 | CHO | 3′-Cl Ph | 5-F | >31.6 | 1.5 | 0.37 |
| 17 | CHO | 4′-Cl Ph | 5-F | >31.6 | 9.09 | 0.29 |
| 18 | CHO | 4′-Cl Ph | 6-F | >31.6 | 9.19 | 0.12 |
| 19 | CHO | 4′-Cl Ph | 5-Cl | >31.6 | 10 | 0.4 |
| 20 | CHO | 4′-Cl Ph | 6-Br | 21 | 32 | 0.09 |
| 21 | CHO | 4′-Cl Ph | 2-O, 6-Cl | 18.6 | 18.2 | 18.2 |
| 22 | CHO | CH (CH3)2 | H | >31.6 | >31.6 | 1.99 |
| 23 | H | Ph | H | >31.6 | >31.6 | >31.6 |
| 24 | H | 4′-Cl Ph | 2-CH2OH, 5-F | 17.4 | 15.1 | 11.5 |
| 25 | CN | 3′-Cl Ph | H | >31.6 | >31.6 | >31.6 |
| 26 | COCH3 | Ph | H | >31.6 | >31.6 | 10 |
| 27 | COCH3 | 2′-Cl Ph | H | >31.6 | >31.6 | >31.6 |
| 28 | COCH3 | 3′-Cl Ph | H | >31.6 | >31.6 | >31.6 |
| 29 | COCH3 | 4′-Cl Ph | H | >31.6 | >31.6 | >31.6 |
| 30 | CH2OH | Ph | H | 14.5 | 0.03 | 0.112 |
| 31 | CH2OH | 3′-F Ph | H | >31.6 | 0.02 | 0.04 |
| 32 | CH2OH | 4′-F Ph | H | 17.0 | 0.03 | 0.118 |
| 33 | CH2OH | 2′-Cl Ph | H | >31.6 | 2.51 | 0.10 |
| 34 | CH2OH | 3′-Cl Ph | H | >31.6 | 0.16 | 0.019 |
| 35 | CH2OH | 4′-Cl Ph | H | >31.6 | 0.079 | 0.016 |
| 36 | CH2OH | 3′-Br Ph | H | >31.6 | 0.032 | 0.028 |
| 37 | CH2OH | 4′-Br Ph | H | >31.6 | 0.39 | 0.031 |
| 38 | CH2OH | 3′-I Ph | H | >31.6 | 0.02 | 0.1 |
| 39 | CH2OH | 3′-CF3 Ph | H | >31.6 | 0.56 | 0.039 |
| 40 | CH2OH | 4′-CF3 Ph | H | >31.6 | 1.25 | 1.25 |
| 41 | CH2OH | 3′-Me Ph | H | >31.6 | 1.00 | 0.031 |
| 42 | CH2OH | 4′-Me Ph | H | >31.6) | 0.15 | 0.031 |
| 43 | CH2OH | 3′-NO2 Ph | H | >31.6 | 0.39 | 0.039 |
| 44 | CH2OH | 4′-NO2 Ph | H | >31.6 | 0.15 | 0.079 |
| 45 | CH2OH | 4′-OMe Ph | H | >31.6 | 0.063 | 0.079 |
| 46 | CH2OH | 2′,4′-2Cl Ph | H | >31.6 | 1.58 | 0.079 |
| 47 | CH2OH | 3′,4′-2Cl Ph | H | >31.6 | 0.31 | 0.031 |
| 48 | CH2OH | 3′,4′-2Br Ph | H | >31.6 | 0.10 | 0.05 |
| 49 | CH2OH | H | 5-F | >31.6 | 13.8 | 16.9 |
| 50 | CH2OH | 3′-F Ph | 5-Cl | 10.0 | 0.16 | 0.07 |
| 51 | CH2OH | 4′-Cl Ph | 5-F | 8.12 | 0.08 | 0.03 |
| 52 | CH2OH | 4′-Cl Ph | 6-F | >31.6 | 1.00 | 0.45 |
| 53 | CH2OH | 4′-Cl Ph | 5-Cl | >31.6 | 13.0 | 5.75 |
| 54 | CH2OH | 4′-Cl Ph | 5-OMe | >31.6 | 12.5 | 1.51 |
| 55 | CH2OH | 4′-Me Ph | 5-F | 11.7 | 0.03 | 0.01 |
| 56 | CH2CH2OH | 4′-Cl Ph | H | >31.6 | >31.6 | >31.6 |
| 57 | CH2CH2CH2OH | Ph | H | >31.6 | >31.6 | >31.6 |
| 58 | C(OH)HCH3 | 4′-Cl Ph | H | >31.5 | 19.9 | 25.1 |
| 59 | CH2OCH3 | 4′-Cl Ph | H | >31.6 | 7.94 | 1.99 |
| 60 |
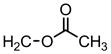
|
4′-Cl Ph | H | >31.6 | 0.063 | 0.039 |
| 61 |
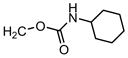
|
4′-Cl Ph | H | >31.6 | 0.2 | 0.02 |
| 62 | COOH | Ph | H | >31.6 | >31.6 | >31.6 |
| 63 | COOH | 4′-Cl Ph | H | >31.6 | >31.6 | >31.6 |
| 64 | COOCH3 | 4′-Cl Ph | H | 17.7 | 25.1 | 31.6 |
| 65 | COOCH3 | 4′-Br Ph | H | 5.00 | 6.30 | 5.00 |
| 66 | CH3 | 4′-Cl Ph | H | >31.6 | >31.6 | >31.6 |
| 67 |
|
4′-Cl Ph | H | 31.6 | 5.01 | 0.10 |
| 68 |
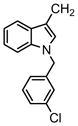
|
3′-Cl Ph | >31.6 | >31.6 | >31.6 | |
| 69 |
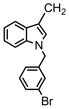
|
3′-Br Ph | >31.6 | >31.6 | >31.6 | |
