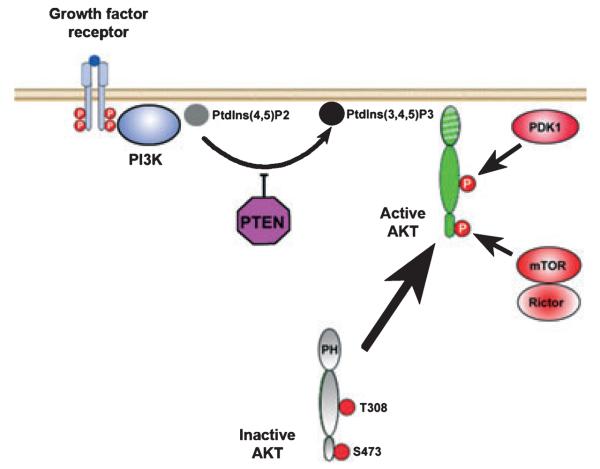
Fig. 4.
Akt signaling pathways. Ligand binding induces autophosphorylation of the cytoplasmic portion of the receptor, resulting in the recruitment and activation of PI3K. PI3K phosphorylates phosphatidylinositol 4,5 biphosphate [PtdIns(4,5)P2] to phosphatidylinositol 3,4,5 triphosphate [PtdIns (3,4,5)P3], which mediates localization of Akt to the inner surface of the cell membrane by interaction with its pleckstrin homology (PH) domain. PTEN acts as a negative regulator of Akt activation. Once localized to the inner surface of the cell membrane, Akt is activated by phosphorylation at two critical residues: Thr308 in the kinase domain and Ser473 in the hydrophobic motif. The Thr308 kinase is 3-phosphoinsitide-dependent kinase 1 (PDK1) (Hanada et al. 2004). The kinase for the Ser473 residue is a complex consisting of mammalian target of rapamycin (mTOR), G protein β-subunit-like protein (GβL), and rictor (Bayascas and Alessi 2005; Sarbassov et al. 2005).