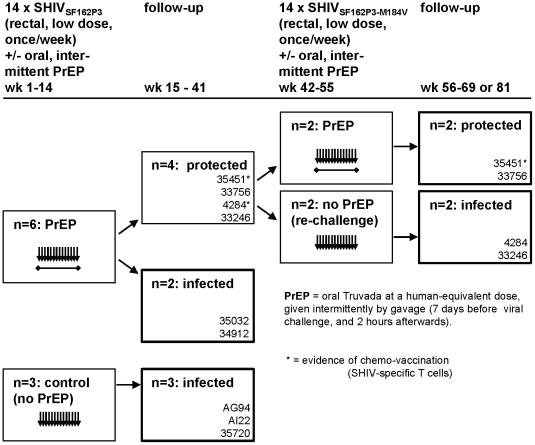
Figure 1. Experimental Design.
SHIV-specific T cells were measured during the indicated experimental procedures. Arrows indicate repeated viral exposures, horizontal lines depict intermittent, oral PrEP. PrEP consisted of human-equivalent doses of oral Truvada. Each virus exposure was flanked by a waning drug dose of 7 days prior, and one drug dose administered 2 hours after exposure, as a model for intermittent PrEP use in humans. Bolded rectangles highlight final outcomes of SHIV challenges. Numbers in lower right corners refer to macaque identifications (IDs).