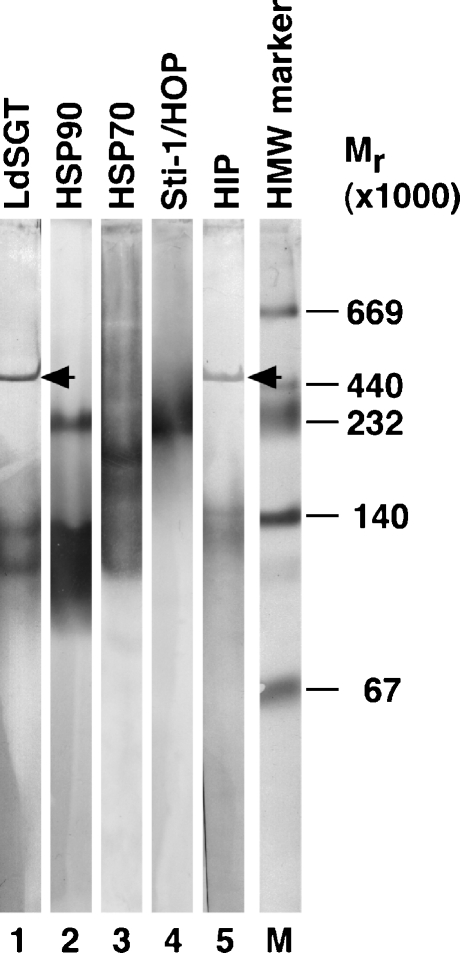
Fig. 6.
Nondenatured size of protein complexes formed by LdSGT and several other putative chaperones and co-chaperones by native gradient gel electrophoresis. Nondenatured proteins from 1 × 107L. donovani wild-type promastigotes were separated in a 0.5× TBE-buffered polyacrylamide gradient gel. The gel was run for 24 h for the complexes to reach their size exclusion limit. The native protein complexes were then denatured, reduced, and blotted onto a PVDF membrane for immunoblot analysis. The blot was probed with antisera against LdSGT (lane 1), HSP90 (lane 2), HSP70 (lane 3), Sti-1 (lane 4), and HIP (lane 5). Protein bands were visualized by alkaline phosphatase reaction. The sizes of marker proteins are indicated to the right of the respective panel (lane M). The arrowheads point at bands at 470 kD. The lanes are derived from one representative immunoblot out of four independent experiments