Abstract
AIM: To access the frequency and level of apoptotic CD34+ cells isolated from the marrow fluid of patients with post-hepatitis cirrhosis.
METHODS: The frequency of bone marrow CD34+ cells and apoptotic bone marrow CD34+ cells in 31 in-patients with post-hepatitis cirrhosis (cirrhosis group), and 15 out-patients without liver or blood disorders (control group) was calculated by flow cytometry. Parameters were collected to evaluate liver functions of patients in cirrhosis group.
RESULTS: The percentage of normal bone marrow CD34+ cells was 6.30% ± 2.48% and 1.87% ± 0.53% (t = 3.906, P < 0.01) while that of apoptotic marrow CD34+ cells was 15.00% ± 15.81% and 5.73% ± 1.57% (t = 2.367, P < 0.05) in cirrhosis and control groups, respectively. The percentage of apoptotic marrow CD34+ cells was 6.25% ± 3.30% and 20.92 ± 18.5% (t = 2.409, P < 0.05) in Child-Pugh A and Child-Pugh B + C cirrhotic patients, respectively. The percentage of late apoptotic marrow CD34+ cells was positively correlated with the total bilirubin and aspartate aminotransferase serum levels in patients with cirrhosis.
CONCLUSION: The status of CD34+ marrow cells in cirrhotic patients may suggest that the ability of hematopoietic progenitor cells to transform into mature blood cells is impaired.
Keywords: Cirrhosis, CD34, Hematopoietic stem cells/Hematopoietic progenitor cells, Apoptosis
INTRODUCTION
Cirrhosis represents the final stage for a wide variety of chronic liver diseases. One of its main clinical manifestations in its decompensatory stage is cytopenia. The causes have been generally recognized as hypersplenism and abnormal bone marrow[1]. However, the more detailed mechanism underlying cytopenia in cirrhotic patients is not clear. An understanding of its mechanism underlying loss of peripheral blood cells would shade further insight into the progress of cirrhosis and its treatment.
Peripheral blood cells are derived from hematopoietic stem cells (HSC). HSC are derived from mesenchymal cells located in wall of yolk sac during the embryonic stage. After establishment of embryonic blood circulation, HSC spread to the liver and hematopoiesis is initiated in the 6th week of embryonic stage and reoccurs in the spleen when hematopoiesis in the liver is decelerated. After birth, the production of blood cells in the liver and spleen dwindles, and almost halts altogether[2]. HSC settle mainly in the bone marrow and produce blood cells for life. As yet, very few studies are available on the phenotypic change of HSC located in bone marrow of cirrhotic patients and its impact on the production of peripheral blood.
CD34, a well known marker for HSC, is a type of phosphoglycoprotein belonging to type 1 trans-membrane protein, helps HSC/hematopoietic progenitor cells (HPC) to adhere to marrow stroma, inhibits differentiation of hematopoietic cells, stimulates formation of HPC, and is involved in intracellular signal transduction, etc. The number of CD34+ cells including hematopoietic cells at several stages is heterogeneous and can differentiate to all blood cell lineages. The CD34 molecule gradually disappears when HSC/HPC differentiate into mature blood cells[3].
In this study, the percentage of apoptotic CD34+ bone marrow cells in patients with post-hepatitis cirrhosis at different stages was calculated by flow cytometry.
MATERIALS AND METHODS
Study subjects
Thirty-one patients with post-hepatitis cirrhosis, admitted to Department of Infectious Diseases, Second Affiliated Hospital of Xi’an Jiaotong University from November 2008 to April 2009, were divided into three groups according to the Child-Pugh classification. The patients did not receive treatment with interferon and only 6 patients were treated with lamivudine or adefovir intermittently before they were enrolled in this study. The control group consisted of 15 patients attending the Outpatient Department of Hematology in our hospital. Bone marrow smears showed that they had no liver and hematological disorders. Laboratory tests were performed to evaluate their liver functions. The clinical details of cirrhosis and control groups are shown in Table 1. Bone marrow was aspirated from each patient. Mononuclear cells (MNC) were isolated from 4 mL freshly-heparinized marrow fluid by Isopaque-Ficoll (Bioer, Hangzhou, China) gradient centrifugation. The study was approved by the local ethical committee, and informed consent was obtained from the patients.
Table 1.
Characteristics of patients in cirrhosis and control groups
| Parameters | Control | Child-Pugh A | Child-Pugh B | Child-Pugh C | P value (cirrhosis vs control) |
| Demographic data | |||||
| Number | 15 | 12 | 11 | 8 | |
| Male/female | 9/6 | 6/6 | 6/5 | 5/3 | |
| Age | 42 (32-53) | 39 (35-50) | 46 (32-60) | 48 (29-60) | NS |
| Lab tests | |||||
| WBC (× 109) | 7.05 ± 2.21 | 4.34 ± 2.01 | 2.93 ± 0.50 | 3.62 ± 1.78 | < 0.01 |
| RBC (× 1012) | 3.53 ± 0.60 | 3.67 ± 0.61 | 3.93 ± 0.75 | 3.34 ± 0.57 | NS |
| PLT (× 109) | 210 ± 105 | 141 ± 114 | 58 ± 42 | 54 ± 27 | < 0.01 |
| ALT(IU/L) | 16 ± 14 | 55 ± 49 | 87 ± 48 | 61 ± 31 | < 0.01 |
| AST(IU/L) | 20 ± 11 | 50 ± 33 | 73 ± 24 | 106 ± 63 | < 0.01 |
| Albumin (g/L) | 43 ± 5 | 40 ± 6 | 35 ± 3 | 30 ± 6 | < 0.01 |
| TBIL (μmol/L) | 10.3 ± 3.5 | 15.8 ± 7.6 | 40.7 ± 23.0 | 78.4 ± 57.8 | < 0.01 |
| γ-GT(IU/L) | 15 ± 12 | 64 ± 63 | 143 ± 171 | 100 ± 108 | < 0.01 |
TBIL: Total bilirubin; NS: No significance.
Flow cytometry evaluation
After Ficoll gradient separation, the MNC were washed with 100 mL of phosphate-buffered saline (PBS), and 106 cells were stained with anti-CD34-FITC MAb (Biosynthesis, Beijing, China) for 30 min at 4°C. Percentage of CD34+ cells was assayed with a Keygen annexin V apoptosis detection kit containing annexin V-PE, propidium iodide (PI; Sigma, US) solution and annexin V binding buffer. CD34+ cells were analyzed to determine the early annexin V+/PI- or late annexin V+/PI+ apoptotic phase.
The MNC were stained again with anti-CD34-FITC MAb, annexin V-PE (Keygen, Nanjing, China) and PI, washed again with PBS and re-suspended in 100 mL of annexin V-PE for 15 min at room temperature. Another 200 mL of binding buffer and 5 mL of PI solution were added and 800 000 cells were obtained by flow cytometry (BD, USA)[4].
Statistical analysis
Statistical analysis was conducted using the SPSS 13.0 statistical package. Difference in independent variables of apoptotic CD34+ cells was detected by t-test and one way ANOVA. P < 0.05 was considered statistically significant.
RESULTS
Percentage of bone marrow CD34+ cells in cirrhotic patients
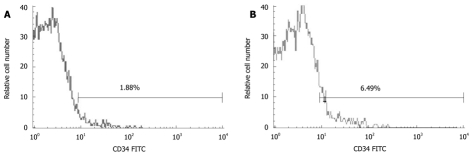
Marrow CD34+ cells were gated from the side scatter height (SSC-H)/CD34 FITC dot plot according to the Milan protocol (Figure 1).
Figure 1.
Flow cytometry showing the percentage of CD34+ cells in controls (A) and cirrhotic patients (B).
The percentage of normal bone marrow CD34+ cells (out of mononuclear cells -CD34+/MNC) was 6.30% ± 2.48% and 1.87% ± 0.53% (t = 3.906, P < 0.01), respectively, in cirrhosis and control groups, while that of CD34+/MNC was 7.01% ± 2.1%, 4.58% ± 2.56%, and 7.72% ± 1.49% (F = 3.586), respectively, in Child-Pugh A-C cirrhosis patients (Figure 2).
Figure 2.
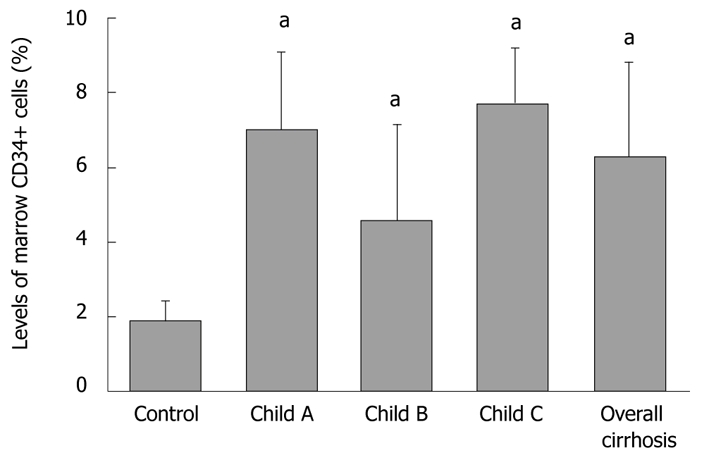
Percentage of bone marrow CD34+ cells in two groups. aP < 0.01 vs control control.
Percentage of apoptotic bone marrow CD34+ cells in cirrhotic patients
The percentage of bone marrow CD34+ cells was determined by FACS analysis with annexin V/PI staining (Figure 3). The percentage of early apoptotic bone marrow CD34+ cells (out of total marrow CD34+ cells) was 6.60% ± 5.83% and 3.88% ± 1.22% (t = 0.912), respectively, while that of late apoptotic bone marrow CD34+ cells was 9.24% ± 13.67% and 1.86% ± 0.86% (t = 2.207, P < 0.05), respectively, in cirrhosis and control groups. The total percentage of apoptotic bone marrow CD34+ (out of total marrow CD34+ cells) cells was 15.00% ± 15.81% and 5.73% ± 1.57% (t = 2.367, P < 0.05), respectively, in cirrhosis and control groups.
Figure 3.
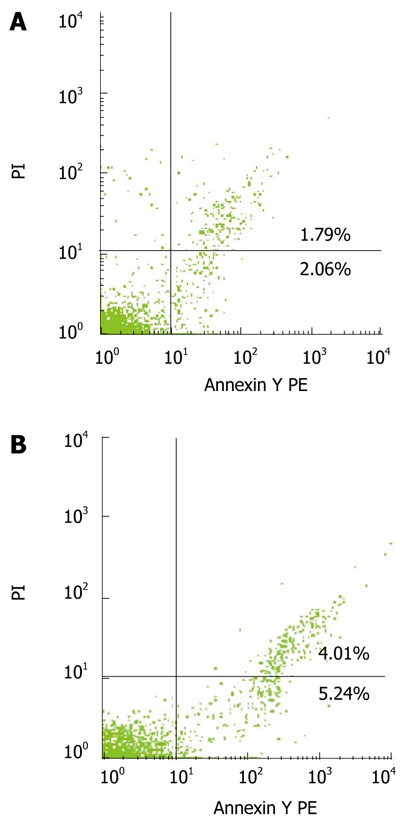
Scatter plot showing percentages of early and late apoptotic bone marrow CD34+ cells in control group (A) and cirrhosis group (B).
The total percentage of apoptotic bone marrow CD34+ cells (out of total marrow CD34+ cells) was 6.25% ± 3.30% and 20.92% ± 18.5% (t = -2.409, P < 0.05), respectively, in early (Child-Pugh A) and late stage (Child-Pugh B+C) cirrhotic patients (Figure 4).
Figure 4.
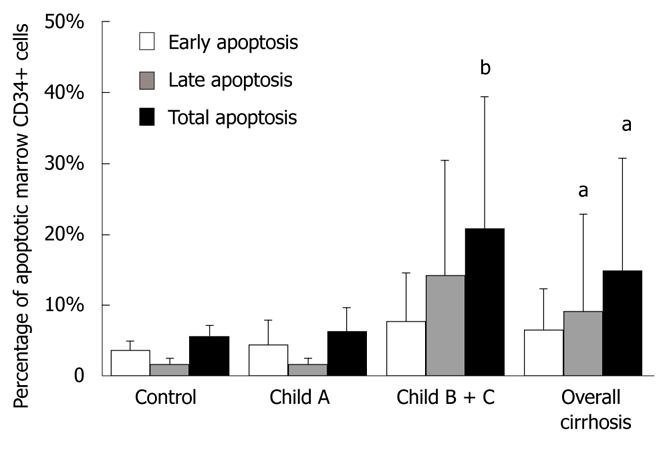
Percentage of apoptotic bone marrow CD34+ cells in controls and Child A, Child B + C cirrhotic patients. aP < 0.05 vs controls, bP < 0.05 vs Child A patients.
Correlation between apoptotic CD34+ bone marrow cells and reduced bilitubin and aminotransferase serum levels
The correlation between apoptotic CD34+ bone marrow cells and reduced bilitubin and aminotransferase serum levels was assessed (Table 2), which showed that the early apoptotic CD34+ bone marrow cells were positively correlated with the serum levels of γ-GT and albumin, while the late apoptotic CD34+ bone marrow cells were negatively correlated with the serum levels of total bilirubin and aspartate aminotransferase (AST) in cirrhotic patients.
Table 2.
Correlation between early, late and total CD34+ cells and laboratory parameters
| Parameters |
Early apoptotic cells |
Late apoptotic cells |
Total CD34+ cells |
|||
| r | P | r | P | r | P | |
| Age | -0.124 | 0.625 | 0.225 | 0.385 | 0.048 | 0.839 |
| RBC | 0.024 | 0.924 | -0.181 | 0.487 | -0.107 | 0.653 |
| WBC | -0.152 | 0.548 | 0.244 | 0.345 | 0.322 | 0.167 |
| PLT | -0.216 | 0.389 | 0.195 | 0.452 | 0.218 | 0.355 |
| TBIL | -0.186 | 0.490 | 0.717 | 0.003b | 0.314 | 0.205 |
| ALT | -0.015 | 0.957 | 0.256 | 0.358 | 0.206 | 0.412 |
| AST | -0.043 | 0.874 | 0.663 | 0.007b | 0.146 | 0.564 |
| γ-GT | -0.117 | 0.667 | 0.136 | 0.630 | 0.471 | 0.048a |
| Albumin | -0.283 | 0.270 | -0.033 | 0.905 | 0.465 | 0.045a |
P < 0.05,
P < 0.01 vs early apoptotic cells.
DISCUSSION
HSC, the ancestors of different blood cells, are multipotent and self-renewable with a great ability to proliferate, and can differentiate into HPC which then differentiate into red blood cells, white blood cells and platelets. HPC, derived from HSC, are not able to renew. The number of HPC is retained by proliferation which expands different mature blood cells[5].
In this study, the expression of CD34 antigen in bone marrows of 31 patients with post-hepatitis cirrhosis was detected by flow cytometry. The percentage of bone marrow CD34+ cells was significantly higher in cirrhosis group than in control group, indicating that bone marrow CD34+ cells are activated in patients with post-hepatitis cirrhosis. HSC/HPC have a compensatory proliferation capacity for cytopenia in cirrhotic patients and enhance their ability to differentiate into mature blood cells, in order to make up the loss of mature blood cells because of hypersplenism, which is significantly different from that for aplastic anemia, in which cytopenia is caused by abnormal HSC/HPC[6].
In this study, the percentage of apoptotic bone marrow CD34+ cells was significantly different in cirrhosis and control groups, and between early and late stage cirrhosis. The percentage of apoptotic CD34+ cells was significantly higher in cirrhosis group than in control group. The percentage of apoptotic bone marrow CD34+ cells (20.92% ± 18.5%) was very high in late stage cirrhotic patients, and almost comparable between early stage cirrhotic patients and controls (6.25% ± 3.30% vs 5.73% ± 1.57%), suggesting that the compensatory ability of HSC/HPC to proliferate is impaired, thus limiting their ability to differentiate into mature blood cells, which may be the cause of cytopenia in late stage cirrhotic patients. This could also explain why the peripheral blood cell count in early stage cirrhotic patients remains normal and the peripheral blood cell count is decreased in late stage cirrhotic patients, although the number of bone marrow CD34+ HPC is still high[7]. Our previous study showed that the Fas expression level is significantly higher in rats with carbon tetrachloride-induced cirrhosis than in normal rats[8]. It is also known that the serum TNF-α and IFN-γ levels are higher in cirrhotic patients, thus up-regulating the expression of Fas, leading to cell apoptosis. We therefore speculate that the TNF-α and IFN-γ/Fas pathways may contribute to the apoptosis of bone marrow HSC/HPC[9-13].
Finally, the correlation between parameters used to evaluate liver functions and apoptotic HSC/HPC was analyzed. The late apoptotic CD34+ bone marrow cells were positively correlated with the serum levels of total bilirubin and AST, indicating that that the impaired function of bone marrow CD34+ cells is correlated with late stage cirrhosis. The establishment of such a correlation can be used in evaluating hematopoiesis in bone marrow of cirrhotic patients.
It has been shown that hypersplenism is the main cause of cytopenia in cirrhotic patients. Decreased production of thrombopoietin in liver may also contribute to thrombocytopenia[14,15]. In this study, the number of HSC/HPC was increased and become more active in cirrhotic patients leading to compensatory effects of peripheral cytopenia. However, as cirrhosis progresses and apoptosis of HSC/HPC increases, hematopoiesis in bone marrow is impaired, eventually leading to disordered homeostasis and poor prognosis, in addition to liver failure and hypersplenism, in end-stage cirrhotic patients. The subjects involved in this study had hepatitis B or hepatitis C-associated cirrhosis. The virological impact on activation of bone marrow CD34 cells and functional impairment therefore merits further investigation.
In conclusion, the percentage of CD34+ bone marrow cells is high in cirrhotic patients, and increased apoptotic CD34+ HSC/HPC are correlated with late stage cirrhosis and total bilirubin and AST serum level.
COMMENTS
Background
Peripheral cytopenia is quite common in cirrhotic patients and the percentage of apoptotic CD34+ cells in bone marrow of cirrhotic patients was calculated.
Research frontiers
The relation between bone marrow CD34+ cells and liver cells has been reported. Bone marrow CD34+ cells can transform into hepatocytes in vitro and in vivo. Meanwhile, autologous or umbilical stem cell transplantation seems to be a promising new therapy for cirrhosis.
Innovations and breakthroughs
Bone marrow CD34+ cells were studied in cirrhotic patients. In this study, the percentage of bone marrow CD34+ cells was high in cirrhotic patients and that the increased apoptotic CD34+ HSC/HPC are correlated with late stage cirrhosis and the total bilirubin and AST serum level.
Applications
The findings in the study can partly explain why peripheral cytopenia occurs in patients with post-hepatitis cirrhosis.
Terminology
HSC: Multipotent stem cells that increase different blood cells including myeloid and lymphoid lineages and are defined by their ability to replenish different blood cells and their ability to self-renew.
Peer review
This is an interesting manuscript describing the role of CD 34+ cells, a well known marker for hematopoietic stem cells, in cirrhotic patients. The flow cytometry evaluation is adequate. The statistical analysis has well been conducted.
Footnotes
Supported by National Natural Science Foundation of China, No.30670961
Peer reviewer: Mariano Malaguarnera, Associate Professor, Department of Internal Medicine and Geriatrics, Catania University, Catania 95124, Italy
S- Editor Sun H L- Editor Wang XL E- Editor Ma WH
References
- 1.Peck-Radosavljevic M. Hypersplenism. Eur J Gastroenterol Hepatol. 2001;13:317–323. doi: 10.1097/00042737-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Péault B. Hematopoietic stem cell emergence in embryonic life: developmental hematology revisited. J Hematother. 1996;5:369–378. doi: 10.1089/scd.1.1996.5.369. [DOI] [PubMed] [Google Scholar]
- 3.Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- 4.Geft D, Schwartzenberg S, Rogowsky O, Finkelstein A, Ablin J, Maysel-Auslender S, Wexler D, Keren G, George J. Circulating apoptotic progenitor cells in patients with congestive heart failure. PLoS One. 2008;3:e3238. doi: 10.1371/journal.pone.0003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnet D. Haematopoietic stem cells. J Pathol. 2002;197:430–440. doi: 10.1002/path.1153. [DOI] [PubMed] [Google Scholar]
- 6.Matsui WH, Brodsky RA, Smith BD, Borowitz MJ, Jones RJ. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia. 2006;20:458–462. doi: 10.1038/sj.leu.2404119. [DOI] [PubMed] [Google Scholar]
- 7.Bashour FN, Teran JC, Mullen KD. Prevalence of peripheral blood cytopenias (hypersplenism) in patients with nonalcoholic chronic liver disease. Am J Gastroenterol. 2000;95:2936–2939. doi: 10.1111/j.1572-0241.2000.02325.x. [DOI] [PubMed] [Google Scholar]
- 8.Dang SS, Biang J, Zhou FL, Gao N, Cheng YA, Li YP. Expressions and significance of Fas antigen on CD34+ cells in experimental cirrhotic rats. Ganzang. 2010;15:40–42. [Google Scholar]
- 9.Alenzi FQ, Al-Ghamdi SM, Tamimi WG, Al-Sebiany AM, El-Nashar IM, El-Tounsi I, Bamaga MS, Al-Enazi MM, Al-Amri AS, Al-Sheikh IH. Apoptosis role of FAS/FAS ligand system in the regulation of myelopoiesis. Yale J Biol Med. 2005;78:25–36. [PMC free article] [PubMed] [Google Scholar]
- 10.Geller J, Petak I, Szucs KS, Nagy K, Tillman DM, Houghton JA. Interferon-gamma-induced sensitization of colon carcinomas to ZD9331 targets caspases, downstream of Fas, independent of mitochondrial signaling and the inhibitor of apoptosis survivin. Clin Cancer Res. 2003;9:6504–6515. [PubMed] [Google Scholar]
- 11.Xiao J, Zou P, Liu Z, Liu L, Hu Z. Selective depletion of the allo-antigen specific T cells by Fas/FasL pathway by cytokine IFN-gamma and IL-2. J Huazhong Univ Sci Technolog Med Sci. 2003;23:344–347. doi: 10.1007/BF02829413. [DOI] [PubMed] [Google Scholar]
- 12.Falasca K, Ucciferri C, Dalessandro M, Zingariello P, Mancino P, Petrarca C, Pizzigallo E, Conti P, Vecchiet J. Cytokine patterns correlate with liver damage in patients with chronic hepatitis B and C. Ann Clin Lab Sci. 2006;36:144–150. [PubMed] [Google Scholar]
- 13.Huerta-Yepez S, Vega M, Garban H, Bonavida B. Involvement of the TNF-alpha autocrine-paracrine loop, via NF-kappaB and YY1, in the regulation of tumor cell resistance to Fas-induced apoptosis. Clin Immunol. 2006;120:297–309. doi: 10.1016/j.clim.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Eissa LA, Gad LS, Rabie AM, El-Gayar AM. Thrombopoietin level in patients with chronic liver diseases. Ann Hepatol. 2008;7:235–244. [PubMed] [Google Scholar]
- 15.Rios R, Sangro B, Herrero I, Quiroga J, Prieto J. The role of thrombopoietin in the thrombocytopenia of patients with liver cirrhosis. Am J Gastroenterol. 2005;100:1311–1316. doi: 10.1111/j.1572-0241.2005.41543.x. [DOI] [PubMed] [Google Scholar]