Figure 7.
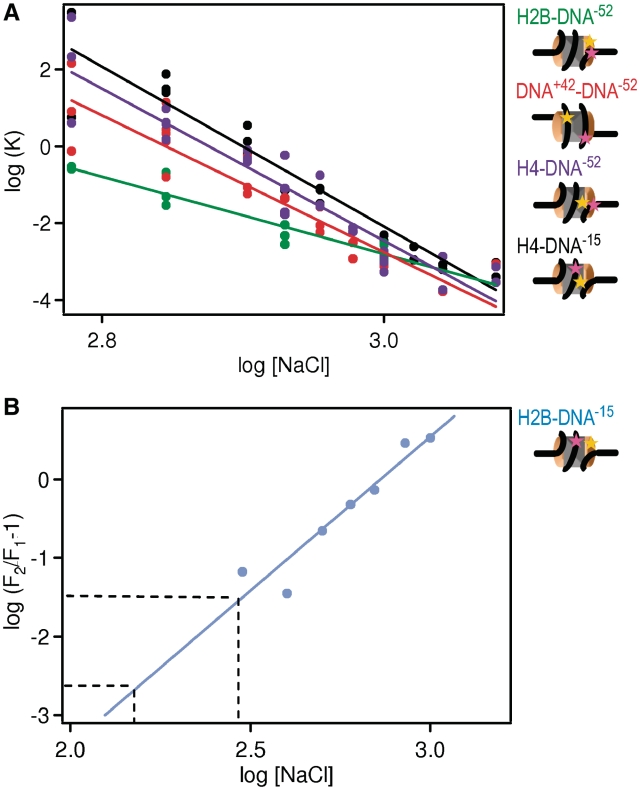
The number of ion pairs between histones and DNA can be derived from the salt dependence of the FRET fraction. (A) Equilibrium constants (K) for histone dissociation as a function of NaCl concentration for H2B–DNA−52 (green), DNA+42–DNA−52 (red), H4–DNA−52 (violet) and H4–DNA−15 (black). Cartoons of nucleosomes indicate the relative locations of labels on the nucleosome. K-values were calculated from the fraction of intact nucleosomes (Figure 5). The number of ions pairs between histones and DNA can be derived from curve fitting (as described in Supplementary Data 2.8, results see Supplementary Table S3). Approximately twice as many ion pairs are broken upon (H3–H4)2 than upon H2A–H2B dissociation. (B) Plot for the determination of the number of ion pairs involved in the opening of the H2A–H2B/(H3–H4)2 interface [see SupplementaryData, Equation (7)]. Data points were calculated from the fraction of intact nucleosomes of H2B–DNA−15 and H2B–DNA−52 (Figure 5). The transition requires the breaking of 4 ± 1 ion pairs as required from the slope of the fit. From extrapolation to physiological salt concentrations (150–300 mM NaCl, indicated by the dashed lines) the fraction of open nucleosomes occupied at physiological salt can be estimated to 0.2–3%.