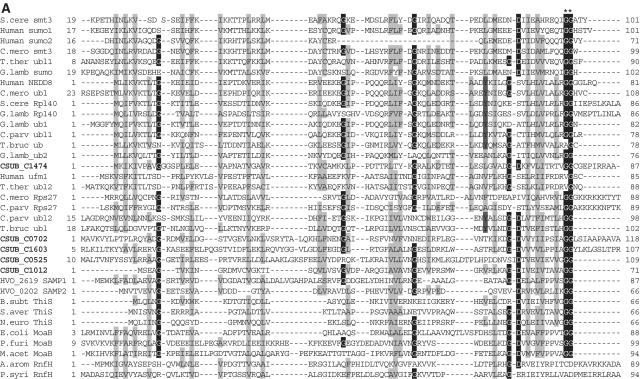
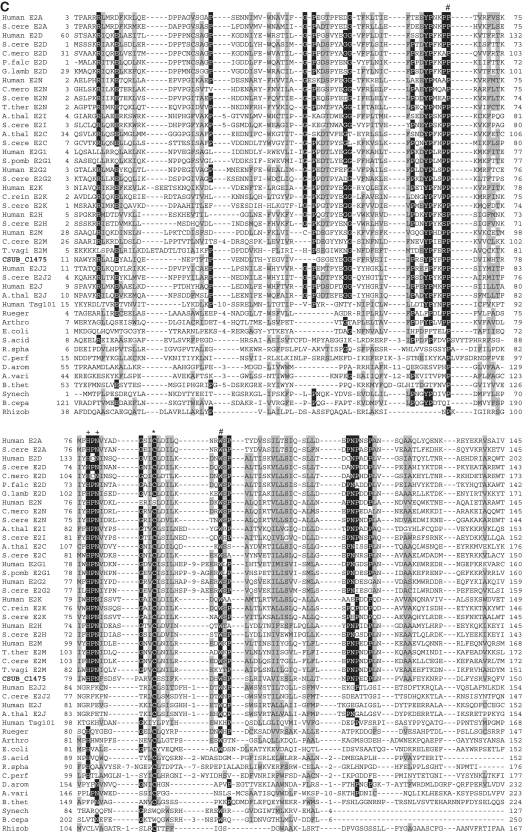
Figure 2.
Sequence alignments of Ub, E1, E2 (super-) and JAMM family proteins. (A) Sequence alignments of eukaryotic and archaeal Ub superfamily proteins; proteins from Saccharomyces cerevisiae; S.cere Smt3 (6320718) and S.cere Rpl40 (6322043), from human; Human sumo2 (54792071), Human sumo1 (54792065), Human NEDD8 (5453760) and Human Ufm1 (7705300), from Cyanidioschyzon merolae; C.mero smt3 (CME004C), C.mero ubl (CML042C) and C.mero Rps27 (CMN125C), from Tetrahymena thermophila; T.ther ubl1 (229594936) and T.ther ubl2 (118367859), from Cryptosporidium parvum; C. parv ubl1 (126654302), C.parv Rps27 (66357428) and C.parv ubl2 (66363058), from Giardia lamblia; G.lamb sumo (159114790), G.lamb Epl40 (159108136), G.lamb ub1 (159112981), G.lamb ub2 (159111413), from Trypanosoma brucei; T.bruc ub (72387960) and T.bruc ubl (72387818), from C. subterraneum; eukaryote-type Ubl (CSUB_C1474) and prokaryote-type Ubls (ThiS/MoaD) (CSUB_C0525, CSUB_C0702, CSUB_C1012, CSUB_C1603), from H. volcanii; SAMPs, HVO_0202 (302595884) and HVO_2619 (302595883), from Bacillus subtilis; B.sub ThiS (CAB13025), from Streptomyces avermitilis; S.aver ThiS (BAC73805), from Nitrosomonas europaea; N.euro ThiS (CAD84196), from Escherichia coli; E.coli MoaB (AAN79339), from Pyrococcus furiosus; P.furi MoaB (1VJK_A), from Methanosarcina acetivorans; M.acet MoaB (AAM05120), from Aromatoleum aromaticum; A.arom NrfH (CAI07579) and from Pseudomonas syringae; P.syri NrfH (AAY39230). Asterisks indicate the C-terminal Gly-Gly motif. (B) Sequence alignments of adenylation and catalytic cysteine domains in E1 superfamily proteins; proteins from human; Human E1L (23510338), Human sumoE1 (60594167), Human UBA1 (23510338), Human UBA2 (4885649), Human UBA3 (38045942), Human UBA5 (13376212), Human ATG7 (119584500) and Human MOCS3 (7657339), from Schizosaccharomyces pombe; S.pomb E1L (162312305) and S.pomb UBA3 (19113852), from S. cerevisiae; S.cere Aos1 (6325438), S.cere UBA1 (6322639), S.cere UBA2 (6320598), S.cere ATG7 (6321965), S.cere UBA4 (6321903) and S.cere YgdLl (6322825), from T. thermophila; T.ther E1L (118383519), T.ther E1B (118351055), T.ther UBA4 (118351953) and T.ther YgdLl (118400480), from Trypanosoma cruzi; T.cruz E1 (71411317), from Plasmodium yoelii; P. yoel UBA2 (82595829) and P.uoel MoeB (83315401), from Trichomonas vaginalis; T.vagi APG7 (123446747), from C. subterraneum; E1l (CSUB_C1476) and MoeB (CSUB_C1135), from H. volacanii; HVO_0558 (292654724), Cupriavidus metallidurans; C.meta ThiF (4039868), from Clostridium perfringens; C.perf (86559649), from Shewanella sp. ANA3; S.ANA3 (117676291), from Rhizobium etli; R.etli (86359719), from Anabaena variabilis; A.vari (ABA25158), from Polaromonas naphthalenivorans; P.naph (121605347), from Nostoc sp. PCC7120; Nostoc (BAB77147), from Xanthomonas axonopodis; X.axon MoeB (21242767), from E. coli; E.coli MoeB (1JW9_B) from C. symbiosum; C.symb ThiF (ABK78649), from P. furiosus; P.furi MoeB (18977661), from Geobacillus kaustophilus; G.kaus MoeBl (56419161), Desulfuromonas acetoxidans; D.acet ThiF (95930339), from Desulfovibrio desulfuricans; D.desu ThiF (78357502), from Bacteroides thetaiotaomicron; B.thet (29349047), from M. tuberculosis; M.tube Rv (15609475), from Cytophaga hutchinsonii; C.hutc (110639176), and from Bacillus thuringiensis; B.thur (110639176). Asterisks and plus indicate adenylation active sites and thiolating cysteine, respectively. Mg2+ chelating motifs (CxxC) are shown by octothorpes. (C) Alignment of E2 superfamily proteins; proteins from human; Human E2A (32967280), Human E2D (5454146), Human E2N (61175265), Human E2G1 (13489085), Human E2G2 (29893557), Human E2K (163660385), Human E2H (4507783), Human E2M (4507791), Human E2J2 (37577124), Human E2J (37577122) and Human Tsg101 (5454140), from Arabidopsis thaliana; A.thal E2I (15230881), A.thal E2C (18403097) and A.thal E2J (18401338), from Chlamydomonas reinhardtii; C.rein E2K (159463008), from C. merolae; C.mero E2D (CMB015C) and C.mero E2N (CMR010C), from Plasmodium falciparum; P.fal E2D (124805463), from S. cerevisiae; S.cere E2A (6321380), S.cere E2D (6319556), S.cere E2N (6320297), S.cere E2I (6320139), S.cere E2C (6324915), S.cere E2G2 (6323664), S.cere E2K (6320382), S.cere E2H (6579192), S.cere E2M (6323337) and S.cere E2J2 (6320947), from S. pombe; S.pomb E2G1 (6323664), from T. thermophila; T.ther E2M (118382495), from T. vaginalis; T.vagi E2M (123484378), from G. lamblia; G. lamb E2D (159111264), from C. subterraneum; CSUB_C1475, from Ruegeria sp; Rueger (22726448), from Arthrobacter sp.; Arthro (A0AW81), from E. coli; E.coli (37927532), from Syntrophus aciditrophicus; S.acid (85859492), from Rhodobacter sphaeroides; R.spha (77387013), from Clostridium perfringens; C.perf (86559649), from Dechloromonas aromatica; D.arom (71847775), from Anabaena variabilis; A.vari (75705484), from Bacteroides thetaiotaomicron; B.thet (29339960), from Synechocystis sp. PCC6803; Synech (38423903), from Burkholderia cepacia; B.cepa (A4JA91), and from Rhizobium sp. NGR234; Rhizob (2496664). Astetisk and octothorpes indicate catalytic cysteine residue and residues forming a conserved stabilizing contact in E2 from eukaryotes, respectively. Flap histidine and asparagine residues are shown by plus. Identical and similar amino acids are shaded in black and gray, respectively. (D) Sequence alignment of JAMM family proteins; proteins from human; Human COPS5 (12654695) and Human PSMD14 (5031981), from A. thaliana; A.thal CSN5A (15219970), from S. cerevisiae; S. cere RPN11 (14318526), from T. brucei; T.bruc RPN11 (18463065) and T.bruc SCN5 (72393165), from G. lamblia; G.lamb RPN11 (159114272), from S. pombe; S.pomb AMSHP (19115685), from C. subterraneum; CSUB_C1473, from Archaeoglobus flugidus; A.flugi JAB (11499780), from Pyrococcus horikoshii; P.hori JAB (3257912), from Pseudomonas aeruginosa; P.aeru JAB (15597298), from Pyrobaculum aerophilum; Py.aer JAB (18313041), from E. coli; E.coli RadC (15801143), from B. subtilis; B.subt RadC (16079856), from M. acetivorans; M.acet RadC (20090827), from Thermotoga maritima; T.mari RadC (15644305), from Aquifex aeolicus; A.aeol (2984019); from Deinococcus radiodurans; D.radi (15805429), from Pseudomonas putida; P.puti (84994017), from Salinibacter rubber; S.rubb (83814538), from M. tuberculosis; M.tube (13880984), from Nocardia farcinica; N.farc (54014564), from Wolinella succinogenes; W.succ, and from Geobacter metallireducens; G.meta. Asterisks indicate the JAMM motif residues. Identical and similar amino acids are shaded in black and gray, respectively.