Introduction
In health care services, technology requires that correct information be duly available to professionals, citizens and authorities, worldwide. Thus, clinical laboratory sciences require standardized electronic exchanges for results of laboratory examinations. Methods. The NPU (Nomenclature, Properties and Units) coding system provides a terminology for identification of result values (property values). It is structured according to BIPM, ISO, IUPAC and IFCC recommendations. It uses standard terms for established concepts and structured definitions describing: which part of the universe is examined, which component of relevance in that part, which kind-of-property is relevant. Unit and specifications can be added where relevant [System(spec) Component(spec); kind-of-property(spec) = ? unit]. Results. The English version of this terminology is freely accessible at http://dior.imt.liu.se/cnpu/ and http://www.labterm.dk, directly or through the IFCC and IUPAC websites. It has been nationally used for more than 10 years in Denmark and Sweden and has been translated into 6 other languages. Conclusions. The NPU coding system provides a terminology for dedicated kinds-of-property following the international recommendations. It fits well in the health network and is freely accessible. Clinical laboratory professionals worldwide will find many advantages in using the NPU coding system, notably with regards to an accreditation process.
Keywords: Patient data, transmission, coding system, online direct access, multilingual
1. Introduction
In applying information technology in health care services, it is essential that the correct information is available at the right time and place to health care professionals, citizens and authorities, worldwide[1, 2]. To reach this aim, clinical laboratory sciences require standardized electronic exchanges regarding the scientific information of examinations. Request transmission, result delivering and patient record storage are now electronically managed and need an adequate nomenclature. Several terminologies now exist to achieve this, such as Euclides, the Finnish terminology, LOINC, READ and NPU. The major advantages of the NPU (Nomenclature for Properties and Units) coding system that it is patient centered, to closely match the clinician’s needs, it is structured according to authoritative international recommendations and codes are long lasting even if techniques change, for a same element and it provides predefined units, avoiding range errors. The constant development required means constant updating and widening to further languages. The updating is driven by the user’s requests as knowledge and practice in clinical laboratory sciences are rapidly expanding. Widening the terminology to as many languages as possible opens the possibility for clinical laboratory professionals of all categories to use it in their native language.
The objective of this contribution is to show that if two laboratories using two different native languages provide data to a same patient file, the overall file will automatically accept the information, through an identical set of codes.
2. Methods
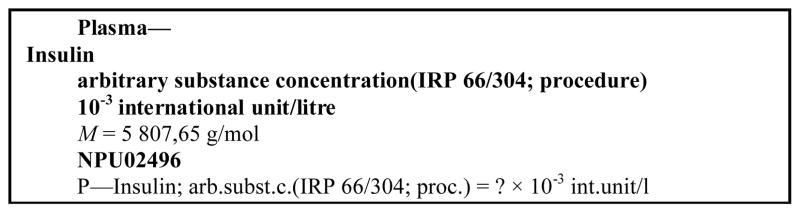
The international NPU coding system, originated from Europe, provides a terminology (so-called “database”) for identification of clinical laboratory result values (property values). It is developed by a joint Committee of IFCC and IUPAC (C-SC-NPU). It is structured according to the Bureau International des Poids et Mesures (BIPM), ISO, International Union of Pure and Applied Chemistry (IUPAC) and International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) recommendations[3, 4]. It uses standard terms for established concepts and structured definitions[5–7], and has been fully described in related works and referenced below[8–13] as follows: (1) which part of the universe is examined, (2) which component of relevance in that part, (3) which kind-of-property is relevant. Unit (4) and specifications can be added where relevant[8, 9]. The resulting syntax and format are displayed in table 1.
Table 1.
Syntax and format of the NPU terminology, with examples
| (1) System(spec)— | (2) Component(spec); | (3) kind-of-property(spec) | (4) = ? unit |
|---|---|---|---|
| P— | Calcium(II); | subst.c.(corr.; proc.) | = ? mmol/l |
| Secr(Bronchus; spec.)— | Mycobacterium; | taxon(proc.) | = ? |
| U— | Ethanol; | subst.c | = ? mmol/l |
| P— | Fibrinogen; | mass c.(imm.; proc.) | = ? g/l |
Each property has a code, the NPU code, giving information on which property is involved, but not on the procedure of the examination[10, 11]. The terminology is centred on the patient: it describes properties of the patient, not of the sample. SI units are used, wherever possible, according to the BIPM recommendations [14]. Component terms refer to authorized sources (CAS for chemical compounds, E.C. for enzymes, Human Genome Nomenclature Database - HUGO - for genes, WHO for reference materials, etc). The terms “component”, “kind-of-property” and “unit” are defined in the VIM.[5].
3. Results
The generic (English) version of the NPU terminology approximately includes 16 000 entries (dedicated kinds-of-property) and covers most fields of clinical biology, eg clinical chemistry, microbiology, virology, parasitology, toxicology, immunology, allergology, molecular biology and genetics, trace elements, reproduction and fertility, thrombosis and haemostasis, transfusion medicine and immunohaematology, pharmacology and doping control.
The English version of this terminology is freely accessible at (http://dior.imt.liu.se/cnpu/) and (http://www.labterm.dk), directly or through the IFCC (http://ifcc.org) and IUPAC (http://iupac.org) websites. It has been nationally used for more than 10 years in Denmark and Sweden. Besides Danish and Swedish, it has been translated into German, Spanish, Catalan, French, Portuguese and Arabic. An example is given in Table 2, showing two possibilities of expression : full* or abbreviated name. The owners of the NPU terminology are the joint IFCC and IUPAC as all publications related to it have been financed by these two organizations.
Table 2.
Example of an entry in the nine cited languages, starting with English
| Country code | NPU code | Entry |
|---|---|---|
| en | NPU02192 | P Glucose; subst.c. = ? mmol/l |
| da | id | P Glucose; stofk. = ? mmol/l |
| *sv | id | Plasma Glukos; substanskoncentration = ? mmol/L |
| *de | id | Plasma Glucose; Stoffkonzentration = ? mmol/l |
| es | id | Pla Glucosa; c.sust. = ? mmol/L |
| ca | id | Pla Glucosa; c.subst. = ? mmol/L |
| fr | id | Pl Glucose; c.mat. = ? mmol/l |
| pt | id | P Glicose; conc.subst. = ? mmol/l |
| ar | id |
|
This terminology is being published in parts, currently[listed in 12, 13].
The use of the NPU coding system is simple as all terms are directly provided by the database for each situation and adapted to it. It is searchable by NPU code, or by component. The result of queries can be downloaded, as well as the entire database. It is continuously being updated according to the evolution of scientific knowledge, technical needs and the newest internationally recognized recommendations. Mapping of the NPU coding system to SNOMED CT is being performed. Notes, explanations and links to various sources of information are also being developed.
Figure 1 shows an example of authoritative source for defining the component “iron” : the Committee on Toxicology of IUPAC Division VII.
Figure 1.
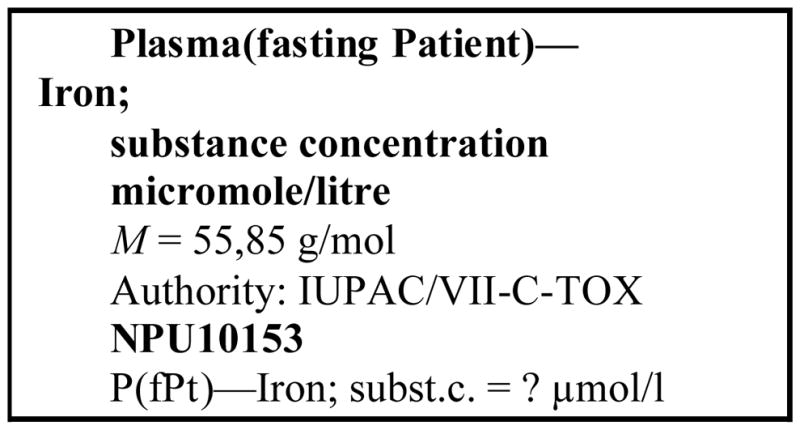
Component structured definition : example of an authoritative source.
Figure 2 shows an example of authoritative source for defining the kind-of-property attached to this component in this case, the WHO reference preparation.
Figure 2.
Kind-of-property structured definition : example of an authoritative source.
The NPU coding system was recommended for national use in Denmark and Sweden in 2001. In these countries, it is the standard coding system for laboratory results in the field of clinical chemistry, and its use in other laboratory fields, especially clinical molecular biology, is increasing.
In Denmark, communication of laboratory results outside the hospitals is managed by the Danish Health Data Network (MedCom), a co-operative venture between authorities, organisations and private firms linked to the Danish healthcare sector. Each year MedCom transmits more than 10 million laboratory requests and reports between Danish laboratories and the EHRs of about 3500 general practitioners (GPs). Over 95% of results transmitted are NPU coded.
Although the NPU terminology is the main coding system, other systems are in use, e.g. for clinical microbiology. MedCom maintains EDIFACT and XML standards for laboratory requests and reports, with slots dedicated for coding system (‘NPU’ or ‘local’), coding system repository (‘Danish Board of Health’ for the NPU system, otherwise local laboratory IDs) and a slot for the actual ‘code value’.
MedCom also supplies a web based facility for on-line requesting of laboratory investigations at the laboratory of choice. Here too, NPU coding is dominant, but codes for local services (local panels, special diagnostic procedures, administrative quirks etc.) are often needed.
A Danish health portal enables GPs to query laboratories nationwide for lab results for specific patients. As most participating laboratories use NPU codes, query results are normally NPU coded. The coding ensures that names and units for the results are shown in a uniform manner according to national agreement.
In other countries, the codes are also transferred via HL7 or OBX-3.
4. Conclusions
The International NPU coding system provides a terminology for dedicated kinds-of-property following the international recommendations for efficient electronic communication in the clinical laboratory sciences. With its ready-to-use codes, it is simple to use and a very good documentation on sources is provided. It fits well in the health network, is freely accessible through several websites and can be used in many countries using the native language. Results are formated in SI units. It is regularly updated and future developments will provide complementary information for a better use of this terminology. Clinical laboratory professionals worldwide will find many advantages in using the NPU coding system, notably with regards to an accreditation process.
References
- 1.Forsum U, Hallander HO, Kallner A, Karlsson D. Impact of Qualitative Analysis in Laboratory Medicine. Trends in Analytical Chemistry. 2005;24:546–555. [Google Scholar]
- 2.Forsum U, Karlsson D. Terminology, categories and representation of examinations in Laboratory Medicine. Clinical Chemistry and Laboratory Medicine. 2005;43:344–345. doi: 10.1515/CCLM.2005.060. [DOI] [PubMed] [Google Scholar]
- 3.Fuentes-Arderiu X. Description of examinations and their results and the standard ISO 15189. Clinical Chemistry and Laboratory Medicine. 2006;44:1164–1167. doi: 10.1515/CCLM.2006.203. [DOI] [PubMed] [Google Scholar]
- 4.ISO 15189:2007, Medical laboratories – Particular requirements for quality and competence
- 5.JCGM 200:2008, International vocabulary of metrology – Basic and general concepts and associated terms (VIM).
- 6.Dybkaer R. An Ontology on Property for physical, chemical, and biological systems. APMIS. 2004;112(Suppl 117):1–210. [PubMed] [Google Scholar]
- 7.Dybkaer R. Description of chemical systems by their properties. Pure and Applied Chemistry. 2008;80:1719–1723. [Google Scholar]
- 8.Olesen H. Properties and units in the clinical laboratory sciences I. Syntax and semantic rules. Pure and Applied Chemistry. 1995;67:1563–1574. [Google Scholar]
- 9.Kenny D, Olesen H. Properties and units in the clinical laboratory sciences II. Kinds-of-property. Pure and Applied Chemistry. 1997;69:1015–1042. [PubMed] [Google Scholar]
- 10.Bruunshuus I, Frederiksen W, Olesen H, Ibsen I. Properties and units in the clinical laboratory sciences: Part III Elements (of properties) and their code values. Pure and Applied Chemistry. 1997;69:2577–2582. [Google Scholar]
- 11.Olesen H, Kenny D, Bruunshuus I, Ibsen I, Jørgensen K, Dybkaer R, Fuentes-Arderiu X, Hill G, Soares de Araujo P, McDonald C. Properties and units in the clinical laboratory sciences: Part IV. Properties and their code values. Pure and Apllied Chemistry. 1997;69:2583–2591. [Google Scholar]
- 12.Soares de Araujo P, Zingales B, Alía-Ramos P, Blanco-Font A, Fuentes-Arderiu X, Mannhalter C, Varming K, Bojesen S, Bruunshuus I, Olesen H. Properties and units in the clinical laboratory sciences: Part XVIII. Properties and units in clinical molecular biology. Pure and Applied Chemistry. 2004;76:1799–1807. [Google Scholar]
- 13.Duffus J, Bruunshuus I, Cornelis R, Dybkaer R, Nordberg M, Kuelpmann W. Properties and units in the clinical laboratory sciences: Part XX. Properties and units in clinical and environmental human toxicology. Pure and Applied Chemistry. 2007;79:87–152. [Google Scholar]
- 14.Bureau international des poids et mesures. Le Systeme international d’unités. The International System of Units. Stedi Media; Paris: 2006. [Google Scholar]