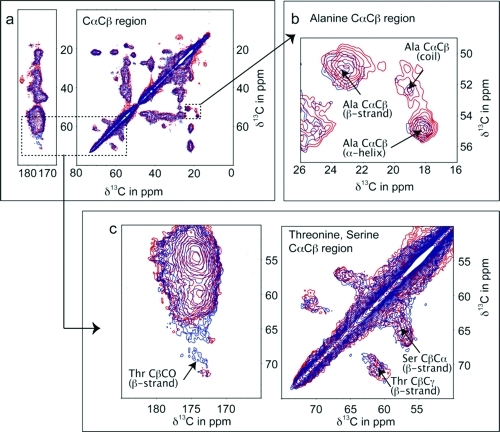
Figure 2.
The CαCβ and CO region of the 13C−13C DARR11 spectra for a uniformly 15N−13C-labeled sample of hVDAC2 shown in red contours, and in the presence of erastin in blue contours (hVDAC2e; 750 MHz AVANCE spectrometer with sample spinning at 14 kHz). Dashed lines represent spectral connectivities of 13Cα−13Cβ and 13CO backbone nuclei of residues with strong dependence on secondary structure, alanine (Cαstrand 50.86 ± 1.28 ppm, Cαhelix 54.86 ± 0.94 ppm, Cαcoil 52.67 ± 1.76 ppm and Cβstrand 21.72 ± 1.77 ppm, Cβhelix 18.27 ± 1.08 ppm, Cβcoil 19.03 ± 1.77 ppm), serine (Cαstrand 57.14 ± 1.11 ppm, Cβstrand 65.39 ± 1.48 ppm), and threonine (Cαstrand 56.28 ± 1.52 ppm, Cβstrand 70.82 ± 2.11 ppm). Common chemical shifts for backbone carbons for these residues based on the structural environment are given in parentheses.(9)