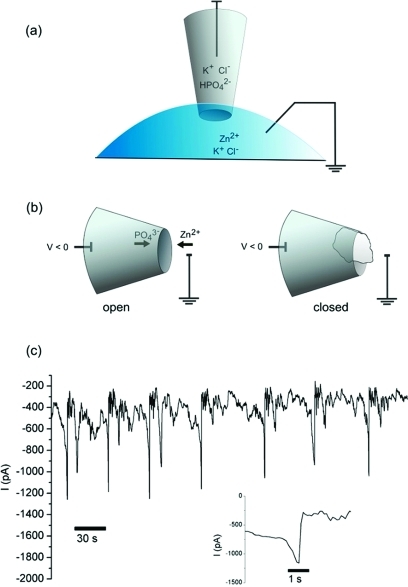
Figure 1.
Measurement of ion current oscillations in a nanopipette. (a) Electrochemical setup to measure ion current through a quartz nanopipette with pore diameter 40−60 nm. All solutions contain KCl (0.1 M) and are buffered at pH 7, with 10 mM potassium phosphate in the barrel and 10 mM Tris-HCl in the bath. Zinc chloride is included in the bath at concentrations of 2−20 μM. (b) Configuration causing ion current oscillations. A negative potential in the nanopipette barrel draws zinc cations from the bath into the pore while phosphate ions are pushed out into the bath. When a precipitate of sufficient size is formed, the pore is blocked and ionic current decreases. (c) Current oscillations in a nanopipette setup with 2 μM zinc chloride in the bath and a potential of −350 mV. Inset: expanded view of one of the open states.