Abstract
Once released, norepinephrine is removed from cardiac synapses via reuptake into sympathetic nerves, whereas transmitter ATP is catabolized by ecto-NTP diphosphohydrolase 1 (E-NTPDase1)/CD39, an ecto-ATPase. Because ATP is known to modulate neurotransmitter release at prejunctional sites, we questioned whether this action may be ultimately controlled by the expression of E-NTPDase1/CD39 at sympathetic nerve terminals. Accordingly, we silenced E-NTPDase1/CD39 expression in nerve growth factor-differentiated PC12 cells, a cellular model of sympathetic neuron, in which dopamine is the predominant catecholamine. We report that E-NTPDase1/CD39 deletion markedly increases depolarization-induced exocytosis of ATP and dopamine and increases ATP-induced dopamine release. Moreover, overexpression of E-NTPDase1/CD39 resulted in enhanced removal of exogenous ATP, a marked decrease in exocytosis of ATP and dopamine, and a large decrease in ATP-induced dopamine release. Administration of a recombinant form of E-NTPDase1/CD39 reproduced the effects of E-NTPDase1/CD39 overexpression. Exposure of PC12 cells to simulated ischemia elicited a release of ATP and dopamine that was markedly increased in E-NTPDase1/CD39-silenced cells and decreased in E-NTPDase1/CD39-overexpressing cells. Therefore, transmitter ATP acts in an autocrine manner to promote its own release and that of dopamine, an action that is controlled by the level of E-NTPDase1/CD39 expression. Because ATP availability greatly increases in myocardial ischemia, recombinant E-NTPDase1/CD39 therapeutically used may offer a novel approach to reduce cardiac dysfunctions caused by excessive catecholamine release.
Introduction
A critical goal in the treatment of myocardial ischemia, congestive heart failure, and hypertension is to reduce excessive sympathetic activation so as to prevent the cardiotoxic effects of norepinephrine (NE) (Kübler and Strasser, 1994; Esler, 2000; Grassi et al., 2009). β-Blockers have been extensively used to alleviate ischemic arrhythmias, preserve left ventricular ejection fraction, and normalize blood pressure (Böhm and Maack, 2000; Lindholm et al., 2005; Zicha et al., 2006). Yet, β-blockers are not without disabling side effects (e.g., fatigue, muscle weakness, cold extremities, nightmares, and impotence) (Böhm and Maack, 2000) and have been shown to increase cardiac sympathetic innervation (Clarke et al., 2010). Thus, a prejunctional inhibition of abnormal NE release may offer advantages over blockade of NE effects at postsynaptic sites. One such means could entail a modulation of NE release by transmitter ATP.
Indeed, NE is coreleased with transmitter ATP from cardiac sympathetic nerves (Burnstock, 2009), and ATP amplifies NE release via a positive feedback process (Sesti et al., 2002); thus, excessive release of NE could be prevented by modulating ATP availability at sympathetic nerve terminals. Unlike NE, which once released is actively taken back into the nerve terminal by a specific transporter (Bönisch and Bruss, 2006), transmitter ATP is instead catabolized by a membrane ATPase (Zimmermann and Braun, 1999; Westfall et al., 2002; Burnstock, 2007). We had previously shown that pharmacological inhibition of the ecto-ATPase ENTPDase1/CD39 potentiates NE release in porcine cardiac tissue and nerve endings isolated from guinea pig hearts (cardiac synaptosomes), whereas the administration of solCD39, a recombinant form of ENTPDase1/CD39, attenuates it (Machida et al., 2005). Likewise, administration of solCD39 attenuates the release of NE in the ischemic guinea pig heart, whereas inhibition of ENTPDase1/CD39 with 6-N,N-diethyl-d-β,γ-dibromomethyleneATP trisodium salt (ARL67156) augments it (Sesti et al., 2003). Collectively, these and other findings suggest that transmitter ATP modulates NE release in an autocrine mode via prejunctional P2X receptors, an action presumably controlled by ENTPDase1/CD39 (Sesti et al., 2002).
To verify the assumption that CD39 modulates NE release, we next used tissues isolated from mice lacking ENTPDase1/CD39. Yet, we found that excessive and prolonged exposure to ATP resulting from ENTPDase1/CD39 deletion caused the desensitization of prejunctional and postjunctional P2X receptors at sympathetic neuro-effector junctions (Schaefer et al., 2007). To obviate this purinergic receptor desensitization, we have now silenced E-NTPDase1/CD39 gene expression in a validated model of peripheral sympathetic neurons, i.e., NGF-differentiated pheochromocytoma PC12 cells (Greene and Tischler, 1976; Taupenot, 2007). These cells express various P2X-receptor isoforms at both the mRNA and protein level (Arthur et al., 2007), yet only P2X1 and P2X3 receptors undergo fast desensitization, whereas P2X2 and P2X4 receptors desensitize slowly and only at much higher ATP concentrations (North, 2002).
We report here that silencing or overexpression of E-NTPDase1/CD39 in NGF-differentiated PC12 cells results in an increase or decrease, respectively, of the ATP-induced release of dopamine, the predominant catecholamine neurotransmitter in these cells (Greene and Tischler, 1976). These novel findings, added to our previous evidence at isolated nerve endings and whole-organ level, clearly demonstrate that E-NTPDase1/CD39 plays a major role at sympathetic nerve terminals as a modulator of the release and effects of ATP and NE. This occurs whether ATP is released by neuronal depolarization or ischemia and therefore has important pathophysiological and pharmacological implications.
Materials and Methods
Cell Culture.
The rat pheochromocytoma PC12 cell line was maintained in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum, 5% donor horse serum, and 1% l-glutamine at 37°C in 5% CO2 (Taupenot, 2007). The differentiating protocol involved plating PC12 cells on tissue culture plates precoated with collagen (rat tail type VII; Sigma-Aldrich, St Louis, MO) and then polylysine (2 μg/ml). Cells were maintained in low serum medium containing 1% fetal bovine serum, 0.5% donor horse serum, and 1% l-glutamine supplemented with 7S-NGF (100 ng/ml) (BD Biosciences Discovery Labware, Bedford, MA) (Morrey et al., 2008). When PC12 cells were infected to overexpress or silence ENTPDase1/CD39 lentivirus was added 72 to 96 h before NGF treatment.
Dopamine and ATP Assays.
PC12 cells were cultured in six-well plates and differentiated with NGF (100 ng/ml) for 3 to 4 days. Dopamine exocytosis was elicited by incubating samples for 10 min with 100 mM K+ (osmolarity was maintained constant by adjusting the NaCl concentration). When pharmacological agents were used, PC12 cells were preincubated for 10 min with given drug concentrations in sodium-Ringer buffer before K+ challenge. The sodium-Ringer composition was 140 mM NaCl, 5 mM KCl, 10 mM HEPES, 1 mM MgCl2, 2 mM glucose, and 2 mM CaCl2. At the end of the 10-min incubation period, supernatants were collected from each well and analyzed for dopamine content by high-performance liquid chromatography with electrochemical detection as described previously (Morrey et al., 2008) with a 10-min retention time. ATP levels were measured with a firefly luciferin-luciferase assay-based commercial kit (ATP Bioluminescence Assay Kit HS II; Roche Diagnostics, Indianapolis, IN). Samples (50 μl) of each supernatant were pipetted into appropriate test tubes, placed in a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA), and processed by autoinjection of 50 μl of luciferin/luciferase reagent. ATP concentrations were calculated from a calibration curve constructed the same day using ATP standards included in the kit. The optimal detection range was between 10−10 and 10−16 mol of ATP. Cell lysates were assayed for protein content by a modified Lowry procedure (Seyedi et al., 2005).
Silencing of ENTPDase1/CD39.
We used short hairpin (sh) siRNA delivered by a modified pCDH1 lentivirus expression vector to knock down the expression of endogenous CD39 in PC12 cells. The cytomegalovirus promoter present in the pCDH1 vector was removed, and the murine RNA polymerase III promoter U6 was cloned by PCR from mouse tail tip genomic DNA inserted in its place to give pCDH1-U6, which retained GFP expression. The U6 promoter was modified to include BbsI and XbaI restriction enzyme sites immediately 3′ of the U6 transcription start site (Yu et al., 2002). BbsI-XbaI-digested pCDH1-U6 was then able to accept annealed oligonucleotides with BbsI and XbaI overhangs that encoded sh siRNAs. We identified potential 19-bp siRNA target sequences within the rat CD39 coding region using the siRNA Target Finder application available at www.genscript.com/ssl-bin/app/rnai (see Materials and Methods). Annealed complementary 55-bp oligonucleotides with BbsI and XbaI overhangs encoding sh siRNAs were ligated into BbsI-XbaI-digested pCDH1-U6. Stbl3 transformants were screened for inserts by ClaI-NheI digest of miniprepped DNA, and select clones were verified by DNA sequencing. A deliberate bp mismatch was introduced in the 5′ sense strand of the sh stem to permit sequencing of the entire sh siRNA insert. Lentiviral particles were prepared and titrated as above. PC12 cells were transduced at a multiplicity of infection of 10. Seventy two hours after transduction cells were harvested, total membranes were prepared, and rat CD39 expression was assayed by Western blot. A lentiviral construct encoding a sh siRNA that did not alter CD39 expression (scrambled) relative to nontransduced or EV-transduced cells was designated as a control virus. A second lentiviral construct, which knocked down CD39 mRNA by more than 90% by reverse transcription-PCR and CD39 protein expression by 80% by Western blot, served as a silencing virus.
Overexpression of ENTPDase1/CD39.
Rat CD39 was cloned from RNA prepared from cultured PC12 cells using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. cDNAs were generated from 2 μg of total RNA using oligo(dT) primers and SuperScript II reverse transcriptase (Invitrogen). Rat CD39 coding sequence was amplified using standard PCR techniques. The final construct included a 5′ EcoRI site, a consensus Kozak sequence followed by a sequence encoding a V5 epitope rat CD39 coding sequence and a 3′ BamHI site. The EcoRI-BamHI-digested PCR product was ligated to similarly digested lentiviral expression vector pCDH1-MCS1-EF-copGFP, and the ligation product was used to transform One Shot Stbl3 Chemically Competent Escherichia coli (Invitrogen). Clones were screened by EcoRI-BamHI double digest of miniprep DNA and verified by complete sequencing of the insert. Lentiviral particles carrying N-terminal V5-tagged rat CD39 were generated following the protocol of Tiscornia et al. (2006) with several exceptions. The packaging vectors psPAX2 and pMD2.G were obtained from Addgene Inc. (Cambridge, MA), and the 293T packaging cells were from GenHunter Corporation (Nashville, TN). The same 293T cells were used to titer virus preparations by fluorescence-activated cell sorting analysis of GFP expression in 293T cells transduced with serial dilutions of the virus preparation. Titers were calculated as transducing units per microliter. Expression of enzymatically active V5-tagged rat CD39 was confirmed by Western blot and apyrase assay of total membranes prepared from transduced 293T cells. For overexpression of rat CD39 in PC12 cells, 50 to 60% confluent cultures were transduced at a multiplicity of infection of 10. Seventy-two hours after transduction, cell membranes were prepared, and expression of protein was assessed by Western blots developed with antibodies to V5 and rat CD39. Apyrase activity present in rat CD39-transduced PC12 cells was compared with that present in membranes prepared from cultures transduced with lentiviral particles lacking the rat CD39 insert.
Western Immunoblotting.
To assess CD39 expression in PC12 cells we used cell membranes. Total membranes were prepared according to the method of Wang et al. (1998). Protein concentration was determined using Bio-Rad Protein Assay Dye Reagent (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. Aliquots of prepared membranes were solubilized in four times lithium dodecyl sulfate sample buffer (Invitrogen) and separated on NuPAGE 4 to 12% gradient gels. Gels were transferred to polyvinylidene difluoride membranes (Invitrogen), blocked for 1 h with 5% nonfat dry milk in phosphate-buffered saline containing 0.05% Tween 20 at room temperature. Membranes were washed with phosphate-buffered saline/0.05% Tween 20 and probed with primary antibody in blocking buffer overnight at 4°C. Membranes were then washed and incubated for 1 h at room temperature with HRP-conjugated secondary antibody in blocking buffer. Membranes were then washed and developed with Immobilon Western Chemiluminescent HRP substrate (Millipore Corporation, Billerica, MA) according to the manufacturer's instructions. Exposures were obtained with Hyperfilm ECL film (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK), scanned, and densitometry performed using ImageJ software (National Institutes of Health, Bethesda, MD). Transferrin receptor (TfR) was used as loading control in all of the Western blots presented.
PCR.
RNA was prepared from PC12 cells and cDNAs were generated as described above. Rat CD39 mRNA target sequences were amplified using a 5′ and 3′ primer pair designed using DNA Star Primer Select software (DNASTAR, Inc., Madison, WI). PCRs were performed with Titanium Taq DNA polymerase (Clontech, Mountain View, CA) according to the manufacturer's directions. The 496-bp reaction product was analyzed by agarose gel electrophoresis. Ethidium bromide-stained bands were visualized on a UV box, and images were obtained with a Polaroid (Minnetonka, MN) camera. Films were scanned and densitometry was performed using ImageJ software. CD39 mRNA expression levels were normalized to β-actin mRNA expression levels obtained in an identical fashion with a 5′ and 3′ primer pair targeting β-actin mRNA sequence.
Induction of Ischemia in PC12 Cells.
Cells were plated and differentiated for 3 to 4 days in precoated T25 flasks. Ischemia was induced by incubating NGF-differentiated PC12 cells for 20 min in glucose-free sodium-Ringer buffer bubbled with 95% N2 and 5% CO2, containing sodium dithionite (3 mM, PO2 ∼0 mm Hg, pH ∼7.3; ischemic release) (Sesti et al., 2003). Control PC12 cells were incubated for an equivalent period of time with oxygenated (95% O2 and 5% CO2) sodium-Ringer buffer. After incubation, supernatants were collected and centrifuged (10 min, 500g, 4°C) and assayed for dopamine and ATP content. Cell lysates were assayed for protein content, and concentrations of both ATP and dopamine were calculated per milligram of protein.
Materials.
Rat tail collagen, poly-l-lysine, nifedipine, ω-conotoxin GVIA, and pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt hydrate (PPADS) were purchased from Sigma-Aldrich. ARL67156 was purchased from A. G. Scientific, Inc. (San Diego, CA). SolCD39 (values for ATP: Km 2.1 μM; Vmax 26 pmol/min; Kcat 260/min) was a generous gift from Drs. C. R. Maliszewski and R. B. Gayle, III (Immunex Corp, Seattle, WA).
Primers.
The rat CD39 primers were 5′-TCAAGGACCCGTGCTTTTACCCAGGATATAAGAAGGTTGT and 3′-GACAGGATGTAGGTCCCCGAGAAACAGTATTCACTCAGGT. The rat β-actin primers were 5′-CTGGCTGGCCGGGACCTGACAGACTAC and 3′-TGGACAGTGAGGCCAGGATAGAGCCACCAA.
Western Blot.
Goat anti-ratCD39 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used at a 1:1000 dilution (WB); murine anti-V5 (Invitrogen) was used at a 1:2500 dilution (WB); HRP-conjugated rabbit anti-goat (Sigma-Aldrich) was used at a 1:40,0000 dilution (WB); HRP-conjugated rabbit anti-mouse (Sigma-Aldrich) was used at a 1:20,000 dilution (WB); and murine anti-human/rat TfR (Zymed Laboratories, South San Francisco, CA) was diluted 1:1500 (WB).
sh siRNA Oligonucleotides.
The target sequence was AAGAAGCTACCGTTTAATCAG. The sh siRNA full sequence was 5′- tttGAAGCTACCC*TTTAATCAGttcaagagaCTGATTAAACGGTAGCTTCttttt and 3′-ctagaaaaaGAAGCTACCGTTTAATCAGtctcttgaaCTGATTAAAG*GGTAGCTT. Uppercase letters indicate the siRNA sequence, and lowercase letters indicate the BbsI and XbaI overhangs and the nine-bp loop sequence. The asterisks denote the mismatch introduced in the sense sequence of the sh siRNA stem to facilitate sequence verification of the construct.
Results
Release of ATP and Dopamine from PC12 Cells Depends on Ca2+ Entry, P2X-Receptor Activation, and E-NTPDase1/CD39 Function.
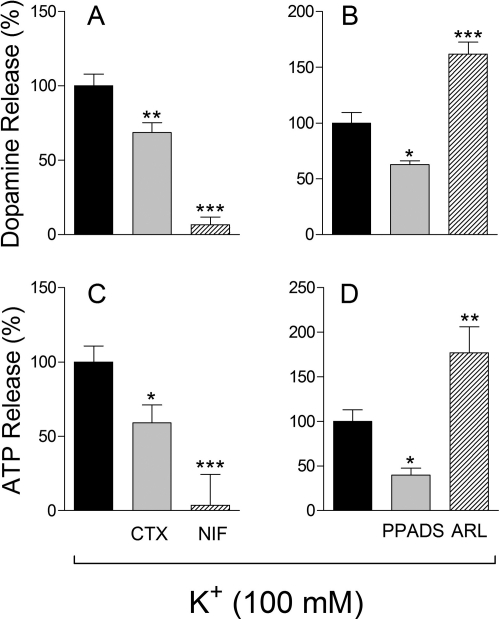
Depolarization of NGF-differentiated PC12 cells with K+ (100 mM) increased the basal release of endogenous ATP and dopamine by ∼2- and ∼3.5-fold, respectively (taken as 100%). In the presence of the N-type Ca2+ channel inhibitor ω-conotoxin GVIA (100 nM) the release of ATP and dopamine was reduced by ∼40 and ∼30%, respectively, whereas in the presence of the L-type Ca2+ channel inhibitor nifedipine (5 μM) the release of both ATP and dopamine was completely abolished (Fig. 1, A and C). This suggested that Ca2+ entry via Ca2+-gated channels is pivotal in the exocytotic process in PC12 cells and the L-type Ca2+ channel seems to play a major role.
Fig. 1.
K+-induced depolarization of NGF-differentiated PC12 cells elicits the release of ATP and dopamine. ATP and dopamine release depends on Ca2+ entry via N- and L-type channels (A and C) and activation of purinergic P2X receptors and catabolism by ENTPDase1/CD39 (B and D). A and C, K+-induced exocytosis of ATP and dopamine and its inhibition by the N-type calcium channel blocker ω-conotoxin GVIA (CTX; 100 nM) or by the L-type calcium channel blocker nifedipine (NIF; 5 μM). B and D, K+-induced exocytosis of ATP and dopamine, its inhibition by the purinoceptor antagonist PPADS (10 μM), at a concentration which selectively blocks P2X receptors (von Kugelgen, 2006) and its potentiation by the ENTPDase1/CD39 inhibitor ARL67156 (ARL; 100 μM). For K+-induced depolarization, cells were incubated with 100 mM K+ for 10 min; ATP and dopamine were assayed at the end of the 10-min incubation period. Bars are means (±S.E.M.; n = 4–19) of percentage increases in neurotransmitter release from basal levels (ATP, 15.3 ± 1.8 nM; dopamine, 2.1 ± 0.2 nM). *, P < 0.05; **, P < 0.01; ***, P < 0.001 from control by one-way ANOVA followed by Dunnett's multiple comparison test.
In the presence of the purinergic receptor antagonist PPADS, at a concentration at which this compound selectively blocks P2X receptors (10 μM) (von Kügelgen, 2006), the K+-induced exocytosis of ATP and dopamine was inhibited by ∼60 and ∼30%, respectively. In contrast, in the presence of the E-NTPDase1/CD39 inhibitor ARL67156 (100 μM) (Crack et al., 1995) the release of ATP and dopamine was enhanced by ∼80 and ∼70%, respectively (Fig. 1, B and D). This suggested that exocytosed ATP activates P2X receptors present on the PC12 cell membrane, eliciting further release of ATP and dopamine and that E-NTPDase1/CD39 also present on the PC12 cell membrane terminates the action of exocytosed ATP.
E-NTPDase1/CD39 Catabolizes ATP Added to PC12 Cells.
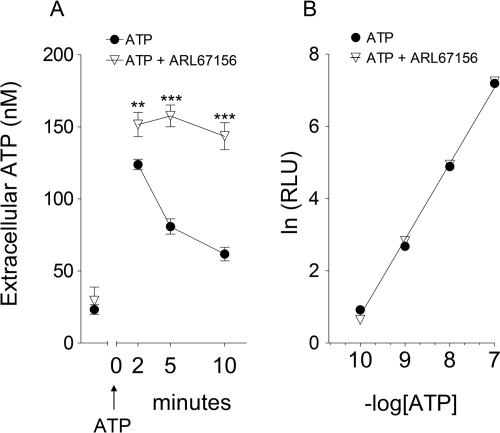
To further support the notion that membrane E-NTPDase1/CD39 modulates the action of ATP in PC12 cells, the cell medium was replaced with sodium-Ringer buffer containing ATP (100 nM), and ATP decay over time was measured. We found that 2 min after the addition of exogenous ATP the extracellular ATP concentration had increased to 125 and 150 nM in the absence and presence of ARL67156 (100 μM), respectively, and then declined to 80 and 65 nM at 5 and 10 min in the absence of ARL67156, respectively, whereas it remained at ∼150 nM in its presence (Fig. 2A). It is noteworthy that at the concentration used (100 μM) ARL67156 did not interfere with the ATP assay. In fact, the concentration-luminescence curve for exogenous ATP was the same in the presence and absence of ARL67156 (100 μM) (Fig. 2B).
Fig. 2.
A, exogenous ATP added to NGF-differentiated PC12 cells is metabolized by ENTPDase1/CD39 present in the cell membrane. Its inhibition is by ARL67156. Cell medium was replaced with sodium-Ringer buffer containing ATP (100 nM) at time 0; extracellular ATP concentration was measured 2, 5, and 10 min after ATP addition both in the absence and presence of ARL67156 (100 μM). Points are mean ATP concentrations (±S.E.M.; n = 6–12). **, P < 0.01; ***, P < 0.001, significantly different from corresponding points in the absence of ARL67156. B, relationship between ATP concentration and luminescence (RLU, relative luminescence) in the absence and presence of ARL67156 (100 μM). ARL67156 does not interfere with the ATP luminescence assay. Points are means (S.E.M. included in each symbol; n = 3). Note that the two lines are superimposable, indicating that ARL67156 does not interfere with the ATP assay.
Silencing and Overexpression of E-NTPDase1/CD39 Influence Catabolism of ATP Added to PC12 Cells.
Because exocytosed ATP seemed to activate P2X receptors on the PC12 cell membrane, eliciting the release of endogenous dopamine, and this action seemed to be modulated by E-NTPDase1/CD39 (Fig. 1B), we questioned whether alteration of E-NTPDase1/CD39 gene expression would reproduce the pharmacological effects. For this, we silenced and overexpressed E-NTPDase1/CD39 in NGF-differentiated PC12 using lentivirus carrying a sh siRNA sequence targeting CD39 mRNA for silencing and rat CD39 coding sequence for overexpression, respectively.
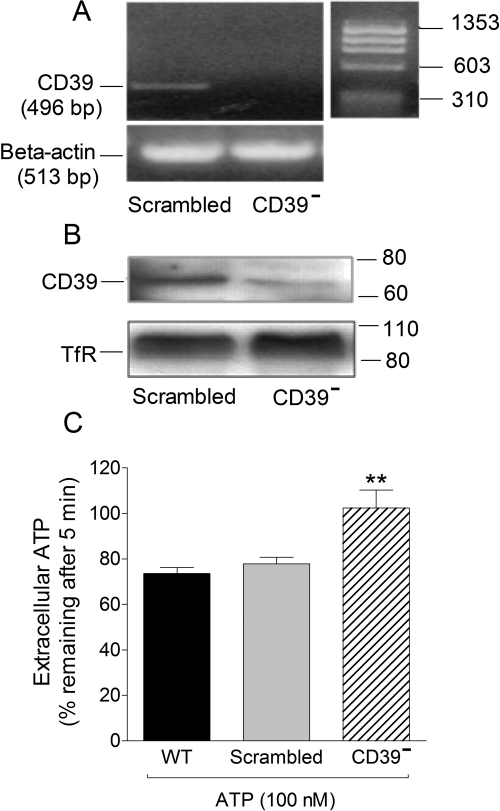
PC12 cells were transduced with lentivirus carrying a sh siRNA targeting a sequence in the CD39 coding region and then differentiated with NGF. PCR and Western blot analysis in differentiated cells revealed the absence of E-NTPDase1/CD39 message and protein (Fig. 3, A and B, respectively). When a small quantity of exogenous ATP (100 nM) was added to E-NTPDase1/CD39-silenced cells (CD39−) to test the effectiveness of the CD39 knockdown treatment, we found that ∼100% of the ATP added remained after 5 min compared with ∼70% in wild-type PC12 cells (Fig. 3C; see also Fig. 2A). No difference was noted between ATP catabolism in WT cells and cells carrying a scrambled sh siRNA sequence (Fig. 3C). Collectively, these findings indicated a markedly decreased ATP metabolism in NGF-differentiated E-NTPDase1/CD39-silenced PC12 cells.
Fig. 3.
Silencing of ENTPDase1/CD39 in NGF-differentiated PC12 cells is associated with a reduced removal of extracellular ATP. A, detection of ENTPDase1/CD39 mRNA by reverse transcription-PCR in differentiated PC12 cells. B, Western immunoblot detecting ENTPDase1/CD39 in differentiated PC12 cells transduced with lentivirus carrying sh siRNA scrambled sequence and its lack in CD39-silenced PC12 cells (CD39−). C, extracellular ATP remaining 5 min after the addition of 100 nM ATP in sodium-Ringer buffer to wild-type cells, cells carrying a scrambled sh siRNA sequence, and CD39-silenced NGF-differentiated PC12 cells. Bars are means (±S.E.M.; n = 12–22). **, P < 0.01 from wild-type cells by one-way ANOVA followed by Dunnett's multiple comparison test.
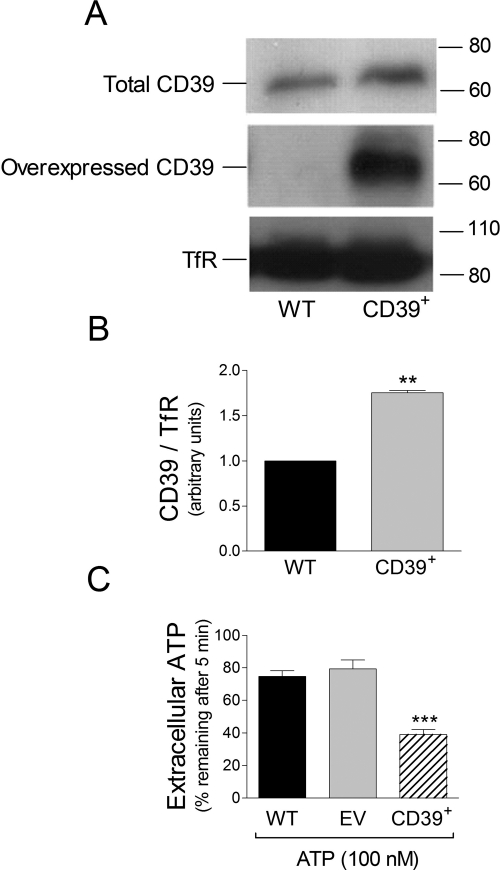
Other PC12 cells were transduced with lentivirus carrying a subcloned rat CD39 coding sequence and then differentiated with NGF. Western blot analysis in differentiated cells revealed a marked increase in total E-NTPDase1/CD39 that was predominantly ascribable to overexpressed E-NTPDase1/CD39 (Fig. 4, A and B). It is noteworthy that when exogenous ATP was added to NGF-differentiated E-NTPDase1/CD39-overexpressing PC12 cells only ∼38% of it remained after 5 min compared with ∼76% in wild-type PC12 cells (Fig. 4D). No difference was noted between ATP metabolism in WT cells and cells infected with lentivirus carrying an EV (Fig. 4C). Collectively, these findings indicated that overexpressed E-NTPDase1/CD39 was metabolically active.
Fig. 4.
Overexpression of ENTPDase1/CD39 in PC12 cells is associated with an enhanced removal of extracellular ATP. A, Western immunoblot detecting total (using antiCD39 antibody; see Materials and Methods) and overexpressed ENTPDase1/CD39 (using an anti-V5 sequence antibody; see Materials and Methods) in membranes prepared from NGF-differentiated PC12 cells. B, quantification of total ENTPDase1/CD39 protein expression in wild-type and CD39+ cells. Bars are means (± S.E.M.; n = 3). **, P < 0.01 from wild-type cells by one-way ANOVA followed by Dunnett's multiple comparison test. C, extracellular ATP remaining 5 min after the addition of 100 nM ATP in sodium-Ringer buffer to NGF-differentiated wild-type cells, NGF-differentiated cells infected with lentivirus carrying an EV, and NGF-differentiated CD39+ PC12 cells. Bars are means (±S.E.M.; n = 4–16). ***, P < 0.001 from wild-type cells by one-way ANOVA followed by Dunnett's multiple comparison test.
Silencing and Overexpression of E-NTPDase1/CD39 Modulate the K+-Induced Release of ATP and Dopamine from PC12 Cells.
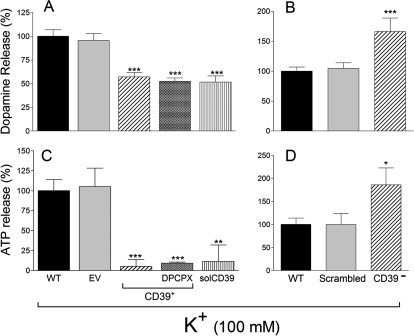
We next investigated ATP and dopamine exocytosis in PC12 cells in which E-NTPDase1/CD39 was either silenced or overexpressed. Upon K+-induced depolarization, the release of ATP and dopamine from NGF-differentiated PC12 cells increased ∼2- and ∼3.5-fold, respectively (taken as 100% in Fig. 5, B and A, respectively). In CD39-overexpressing cells, K+-induced ATP and dopamine release were reduced by ∼90 and ∼40%, respectively. A ∼90 and ∼50% decrease in ATP and dopamine release, respectively, was also observed in CD39-overexpressing cells treated with the selective adenosine A1-receptor antagonist DPCPX (100 nM) (Koyama et al., 2003), excluding an adenosine-induced inhibition of ATP and dopamine release. A similar reduction of ATP and dopamine release was observed when a soluble form of E-NTPDase1/CD39 (solCD39, 10 μM; Kcat 260/min) (Gayle et al., 1998; Sesti et al., 2002) was added to wild-type cells; in this case, K+-induced ATP and dopamine exocytosis decreased by ∼90 and ∼50%, respectively (Fig. 5, C and A, respectively). In contrast, in E-NTPDase1/CD39-silenced PC12 cells, K+-induced ATP and dopamine exocytosis increased by ∼100 and ∼85%, respectively (Fig. 5, D and B, respectively). Although our findings obtained with CD39 silencing clearly show an effect of this enzyme on ATP and dopamine release, it is conceivable that other ectonucleotidases, such as NTPDase2 and NTPDase3, might contribute to extracellular ATP hydrolysis by PC12 cells (Vollmayer et al., 2001).
Fig. 5.
K+-induced depolarization of NGF-differentiated PC12 cells elicits the release of ATP and dopamine, which is attenuated in CD39-overexpressing PC12 cells. The addition of solCD39 mimics the effects of ENTPDase1/CD39 overexpression. In contrast, CD39-silencing enhances the depolarization-induced release of ATP and dopamine. For K+-induced depolarization cells were incubated with 100 mM K+ for 10 min; ATP and dopamine were assayed at the end of the 10-min incubation period. Bars are means (±S.E.M.; n = 3–34) of percentage increases in ATP (C and D) and dopamine (A and B) release over control from basal levels (ATP, 18.0 ± 2.0 nM; dopamine, 7.7 ± 1.8 nM). *, P < 0.05; **, P < 0.01; ***, P < 0.001 from wild-type control by one-way ANOVA followed by Dunnett's multiple comparison test.
Silencing and Overexpression of E-NTPDase1/CD39 Modulate the ATP-Induced Release of Dopamine from PC12 Cells.
Having ascertained that the expression level of E-NTPDase1/CD39 modulates ATP and dopamine exocytosis in PC12 cells, we next investigated whether altering CD39 expression would affect ATP-induced dopamine release in PC12 cells. The administration of exogenous ATP to wild-type NGF-differentiated PC12 cells elicited a concentration-dependent increase in dopamine release in the ATP range of 3 to 100 μM. In the presence of PPADS, at a selective P2X-receptor-blocking concentration (10 μM) (von Kügelgen, 2006), this concentration-response curve was shifted downward, indicating that ATP elicits the release of dopamine by activating P2X receptors (Fig. 6A). The administration of ATP to CD39-silenced or CD39-overexpressing NGF-differentiated PC12 cells resulted in an increase or decrease in dopamine release, respectively (Fig. 6B).
Fig. 6.
A, concentration-response curves for ATP-induced dopamine release in NGF-differentiated PC12 cells: inhibition by PPADS (10 μM), i.e., at a concentration that selectively blocks P2X receptors (von Kugelgen, 2006). Points are percentage increases in dopamine release above control (means ± S.E.M.; n = 3–12). **, P < 0.01; ***, P < 0.001 from corresponding control point by unpaired t test. B, ATP-induced dopamine release is attenuated by overexpression of ENTPDase1/CD39 and potentiated in CD39-silenced cells. Basal dopamine release in WT cells was 9.9 ± 2.6 nM (±S.E.M.; n = 4–11). *, P < 0.05; **, P < 0.01; ***, P < 0.001 from their own control by unpaired t test.
Silencing and Overexpression of E-NTPDase1/CD39 Modulate the Ischemia-Induced Release of ATP and Dopamine from PC12 Cells.
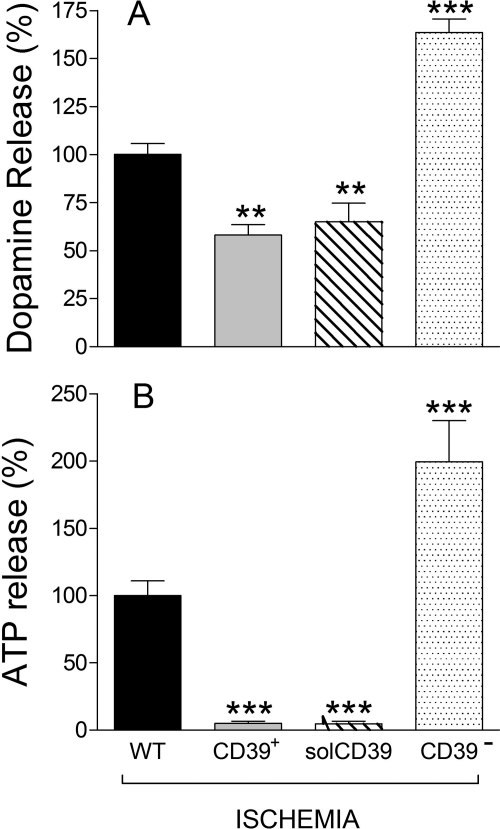
Inasmuch as ATP is released from cardiac neurons under ischemic conditions (Sesti et al., 2003), we next assessed whether alteration of the E-NTPDase1/CD39 gene would modulate ischemia-induced ATP release and its neurotransmitter-releasing effects. After 20 min of simulated ischemia, the release of ATP and dopamine from NGF-differentiated PC12 cells increased by ∼50 and ∼200%, respectively (taken as 100% in Fig. 7). In contrast, ischemia-induced ATP release was practically abolished in E-NTPDase1/CD39-overexpressing cells or wild-type cells treated with solCD39 (10 μM) (Fig. 7B), whereas the enhancement in dopamine release was reduced by ∼40% (Fig. 7A). Remarkably, in E-NTPDase1/CD39-silenced PC12 cells (CD39−), ischemia induced a large increase in both ATP and dopamine release (Fig. 7).
Fig. 7.
Release of dopamine (A) and ATP (B) from NGF-differentiated PC12 cells exposed to ischemia for 20 min. The effect of overexpression or silencing of ENTPDase1/CD39 and comparison with the effect induced by the administration of solCD39 to WT cells (10 μM; solCD39 catalytic activity for ATP: Km 2.1 μM; Vmax 26 pmol/min; Kcat 260/min) are shown. ATP and dopamine were assayed at the end of the 20-min ischemic period. Bars are means (±S.E.M.; n = 4–20) of percentage changes in ATP and dopamine release from control (i.e., WT). Basal ATP and dopamine release was 11.8 ± 2.3 and 4.4 ± 0.9 nM. **, P < 0.01; ***, P < 0.001 from control (i.e., WT) by one-way ANOVA with Dunnett's multiple comparison test.
Discussion
The purpose of this study was to answer important outstanding questions on the likely role of E-NTPDase1/CD39 as a modulator of the autocrine effects of transmitter ATP at sympathetic nerve terminals. For this, we investigated the release of ATP and dopamine in a model of peripheral sympathetic neuron with targeted deletion of E-NTPDase1/CD39. Our findings indicate that the level of expression of E-NTPDase1/CD39 modulates the autocrine effects of transmitter ATP, thus exerting a key influence on the release of catecholamines from sympathetic terminals, in both normal and ischemic conditions.
We chose a phenotypically established model of sympathetic neuron, the NGF-differentiated rat pheochromocytoma PC12 cell line (Greene and Tischler, 1976; DiMaggio et al., 1994; Taupenot, 2007). In fact, these cells are known to express purinergic P2X receptors (Arthur et al., 2007), store ATP and catecholamines in dense-core vesicles (Fabbro et al., 2004), express E-NTPDase1/CD39 (Vollmayer et al., 2001), and release ATP and dopamine upon depolarization (Gardner et al., 2005). As observed before by others (Avidor et al., 1994), we noted that ATP and dopamine exocytosis in differentiated PC12 cells depends on Ca2+ entry via voltage-gated Ca2+ channels, particularly of the L-type. It is noteworthy that we found that, similar to sympathetic nerve endings (i.e., cardiac synaptosomes) (Sesti et al., 2002), transmitter ATP modulated in an autocrine mode its own release and that of dopamine by activating P2X receptors on the cell surface, as indicated by the finding that P2X-receptor blockade with PPADS, at a concentration selective for this purinergic receptor class (10 μM) (von Kügelgen, 2006), attenuated not only the release of ATP, but that of dopamine as well. Moreover, analogous to what we had seen in cardiac synaptosomes (Sesti et al., 2002), inhibition of E-NTPDase1/CD39 with ARL67156 (Crack et al., 1995) potentiated the release of both ATP and dopamine. This indicates that inhibition of E-NTPDase1/CD39 promotes the exocytotic effects of ATP.
This view is now clearly demonstrated in PC12 cells in which E-NTPDase1/CD39 was silenced by siRNA technology, as proven at message, protein, and activity levels (see Fig. 3). Indeed, in E-NTPDase1/CD39-silenced cells, depolarization-induced ATP release was much greater than in wild-type control cells. Moreover, in PC12 cells overexpressing E-NTPDase1/CD39, as proven at the protein and activity levels (Fig. 4), depolarization-induced ATP release was much less than in wild-type controls (Fig. 5). This reflected not only an enhanced ATP metabolism, but likely also a weaker P2X-receptor-mediated positive feedback, as indicated by the associated decrease in dopamine release. Indeed, the addition of solCD39 to wild-type control cells practically abolished the depolarization-induced ATP release.
That modulation of ATP release via E-NTPDase1/CD39 has an important influence on the autocrine release of other transmitters, such as dopamine, is clearly shown by our findings in PC12 cells genetically deprived of E-NTPDase1/CD39 or overexpressing it. Indeed, in E-NTPDase1/CD39-silenced cells the much increased ATP availability was accompanied by a major increase in dopamine release, whereas in cells overexpressing E-NTPDase1/CD39, or in wild-type cells treated with solCD39, the diminished availability of transmitter ATP was associated with a reduced dopamine release. It is noteworthy that in cells overexpressing CD39, or treated with solCD39, the decrease in ATP release was much greater than that of dopamine (Figs. 5 and 7). This difference probably depends on the fact that dopamine release does not rely solely on ATP-induced potentiation and thus is not completely abolished in the absence of extracellular ATP.
The relevance of purinergic ionotropic receptors to the release of dopamine by transmitter ATP is proven by the fact that the magnitude of dopamine release directly depended on the concentration of exogenous ATP administered to PC12 cells and that pharmacologic blockade of P2X receptors markedly diminished the dopamine-releasing effect of ATP (Fig. 6). In fact, the relationship between exogenous ATP concentration and magnitude of dopamine release was also governed by E-NTPDase1/CD39 availability. The lower the availability of E-NTPDase1/CD39, as in the CD39-silenced cells, the greater the magnitude of dopamine release; and, conversely, the higher the availability of E-NTPDase1/CD39, as in CD39-overexpressing cells, the lower the magnitude of dopamine release. It is noteworthy (Fig. 6B) that we found that the increase in ATP-induced dopamine release from CD39-silenced cells and the decrease in ATP-induced dopamine release from CD39-overexpressing cells both declined with increasing exogenous ATP concentrations. This suggests that P2X receptors mediating the ATP-induced release of dopamine may have become progressively desensitized with increasing ATP concentrations (Werner et al., 1996; North, 2002; Schaefer et al., 2007).
Having established an inverse relationship between E-NTPDase1/CD39 availability and neurotransmitter release in physiological conditions, such as neuronal depolarization, we questioned whether this relationship would also apply to pathophysiological conditions, such as ischemia, which is known to be accompanied by an increased release of ATP from sympathetic nerve endings and cardiac myocytes (Vassort, 2001; Sesti et al., 2003) and by an increase in E-NTPDase1/CD39 expression and activity in the heart (Kittel et al., 2005). Indeed, we found that a 20-min exposure of PC12 cells to ischemic conditions greatly magnified the release of both ATP and dopamine (Fig. 7). This response was increased in E-NTPDase1/CD39-silenced cells and markedly reduced in cells overexpressing E-NTPDase1/CD39 or wild-type cells treated with solCD39. Thus, E-NTPDase1/CD39 is pivotal in regulating the magnitude of ATP released by ischemia from cells bearing a sympathetic phenotype and, in turn, in modulating the release of dopamine from the same cells. Inasmuch as catecholamine and ATP release is greatly increased in myocardial ischemia, an augmented catabolism of released ATP by E-NTPDase1/CD39 is likely to be cardioprotective in ischemia/reperfusion, as reported by Köhler et al. (2007).
The decrease in neurotransmitter release from cells overexpressing CD39, or treated with solCD39 (Fig. 5), may not be caused solely by ATP removal but conceivably also by an increased formation of adenosine by ecto-5′-nucleotidase (CD73) and the consequent activation of inhibitory adenosine A1 receptors. Yet, although A1 receptors have been shown to be present on PC12 cells (Pingle et al., 2007), no CD73 activity or adenosine formation have been detected in these cells (Joseph et al., 2004). Indeed, we found that the A1-receptor antagonist DPCPX (Koyama et al., 2003) did not modify the K+-induced release of ATP and dopamine in CD39-overexpressing PC12 cells (Fig. 5).
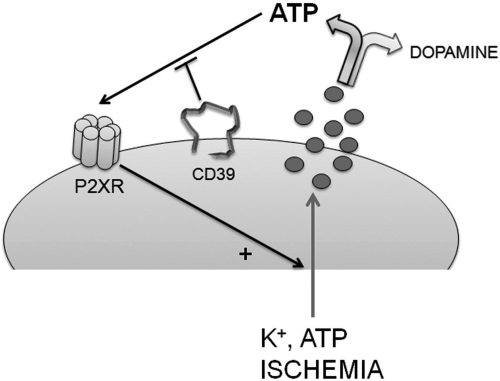
In conclusion (Fig. 8), our findings indicate that membrane depolarization and ischemia each promotes the release of neurotransmitter ATP and dopamine from PC12 cells that have acquired a sympathetic nerve ending phenotype after NGF treatment. Once released, ATP functions in an autocrine mode to activate ionotropic P2X purinergic receptors on the cell membrane, thus eliciting further exocytosis of ATP and dopamine. The autocrine response to released ATP depends on the concentration of ATP at P2X receptors. Because released ATP is catabolized by E-NTPDase1/CD39 at the cell membrane, the activity of this enzyme determines the concentration of ATP and, thus, the magnitude of the P2X receptor-mediated positive-feedback mechanism that magnifies the release of ATP and dopamine. Although we did not attempt to identify which P2X receptor isoforms may contribute to this positive-feedback mechanism, all isoforms are capable of increasing Ca2+ inflow through the neuronal membrane (North, 2002; Jarvis and Khakh, 2009), thus potentiating neurotransmitter release. Although the present study implies that P2X receptors were responsible for the ATP-mediated effects, additional studies suggest that P2Y receptors increase intracellular Ca2+ concentrations in PC12 cells (Moskvina et al., 2003; Lechner et al., 2004) and might therefore participate in the autocrine response to released ATP.
Fig. 8.
The level of CD39 expression and activity modulates neurotransmitter release from sympathetic nerve endings. The scheme shows that depolarization (e.g., by K+ or ATP) and ischemia elicit the release of ATP and catecholamine neurotransmitters (i.e., dopamine) from a model of sympathetic nerve terminal (i.e., PC12 cells). Released ATP activates ionotropic P2X receptors, furthering the release of ATP and dopamine by an action that is controlled by the catabolic action of E-NTPDase1/CD39.
Sympathetic hyperinnervation, enhanced adrenergic activity, and excessive catecholamine release are recognized causes of cardiac dysfunction and arrhythmias in myocardial ischemia (Chen et al., 2001; Selwyn and Braunwald, 2001; Hasan et al., 2006), congestive heart failure (Benedict et al., 1996; Cao et al., 2000; Esler and Kaye, 2000), and hypertension (Julius and Nesbitt, 1996; Esler, 2000; Petersson et al., 2002). Thus, our findings identify a new protective role for neuronal E-NTPDase1/CD39, i.e., limitation of exaggerated catecholamine release in all of these conditions. This is in addition to the role of E-NTPDase1/CD39 as the main control system for blood fluidity (Marcus et al., 1997, 2003; Fung et al., 2009).
Acknowledgments
We thank Dr. Samie Jaffrey for helpful suggestions.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL034215, HL046403, HL047073, HL089521]. A.J.M. is the recipient of a MERIT Review Grant from the Department of Veterans' Affairs and a grant from the Cancer Research and Treatment Fund.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.179994.
- E-NTPDase1
- ecto-NTP diphosphohydrolase 1
- NE
- norepinephrine
- GFP
- green fluorescent protein
- bp
- base pair
- NGF
- nerve growth factor
- ANOVA
- analysis of variance
- WT
- wild type
- PPADS
- pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt hydrate
- PCR
- polymerase chain reaction
- sh
- short hairpin
- siRNA
- small interfering RNA
- ARL67156
- 6-N,N-diethyl-d-β,γ-dibromomethyleneATP trisodium salt
- DPCPX
- 8-cyclopentyl-1,3-dipropylxanthine
- HRP
- horseradish peroxidase
- WB
- Western blot
- TfR
- transferrin receptor
- EV
- empty vector.
Authorship Contributions
Participated in research design: Corti, Olson, Marcus, and Levi.
Conducted experiments: Corti and Olson.
Contributed new reagents or analytic tools: Olson and Marcus.
Performed data analysis: Corti and Levi.
Wrote or contributed to the writing of the manuscript: Corti, Olson, Marcus, and Levi.
References
- Arthur DB, Taupenot L, Insel PA. (2007) Nerve growth factor-stimulated neuronal differentiation induces changes in P2 receptor expression and nucleotide-stimulated catecholamine release. J Neurochem 100:1257–1264 [DOI] [PubMed] [Google Scholar]
- Avidor B, Avidor T, Schwartz L, De Jongh KS, Atlas D. (1994) Cardiac L-type Ca2+ channel triggers transmitter release in PC12 cells. FEBS Lett 342:209–213 [DOI] [PubMed] [Google Scholar]
- Benedict CR, Shelton B, Johnstone DE, Francis G, Greenberg B, Konstam M, Probstfield JL, Yusuf S. (1996) Prognostic significance of plasma norepinephrine in patients with asymptomatic left ventricular dysfunction. SOLVD Investigators. Circulation 94:690–697 [DOI] [PubMed] [Google Scholar]
- Böhm M, Maack C. (2000) Treatment of heart failure with β-blockers. Mechanisms and results. Basic Res Cardiol 95:15–24 [DOI] [PubMed] [Google Scholar]
- Bönisch H, Brüss M. (2006) The norepinephrine transporter in physiology and disease. Handb Exp Pharmacol 175:485–524 [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797 [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2009) Autonomic neurotransmission: 60 years since sir Henry Dale. Annu Rev Pharmacol Toxicol 49:1–30 [DOI] [PubMed] [Google Scholar]
- Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, et al. (2000) Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 101:1960–1969 [DOI] [PubMed] [Google Scholar]
- Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. (2001) Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res 50:409–416 [DOI] [PubMed] [Google Scholar]
- Clarke GL, Bhattacherjee A, Tague SE, Hasan W, Smith PG. (2010) β-Adrenoceptor blockers increase cardiac sympathetic innervation by inhibiting autoreceptor suppression of axon growth. J Neurosci 30:12446–12454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack BE, Pollard CE, Beukers MW, Roberts SM, Hunt SF, Ingall AH, McKechnie KC, IJzerman AP, Leff P. (1995) Pharmacological and biochemical analysis of FPL 67156, a novel, selective inhibitor of ecto-ATPase. Br J Pharmacol 114:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaggio DA, Farah JM, Jr, Westfall TC. (1994) Effects of differentiation on neuropeptide-Y receptors and responses in rat pheochromocytoma cells. Endocrinology 134:719–727 [DOI] [PubMed] [Google Scholar]
- Esler M. (2000) The sympathetic system and hypertension. Am J Hypertens 13:99S–105S [DOI] [PubMed] [Google Scholar]
- Esler M, Kaye D. (2000) Measurement of sympathetic nervous system activity in heart failure: the role of norepinephrine kinetics. Heart Fail Rev 5:17–25 [DOI] [PubMed] [Google Scholar]
- Fabbro A, Skorinkin A, Grandolfo M, Nistri A, Giniatullin R. (2004) Quantal release of ATP from clusters of PC12 cells. J Physiol 560:505–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung CY, Marcus AJ, Broekman MJ, Mahaut-Smith MP. (2009) P2X(1) receptor inhibition and soluble CD39 administration as novel approaches to widen the cardiovascular therapeutic window. Trends Cardiovasc Med 19:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, Westfall TC, Macarthur H. (2005) Endothelin-1-induced inhibition of ATP release from PC-12 cells is mediated by the ETB receptor: differential response to endothelin-1 on ATP, neuropeptide Y, and dopamine levels. J Pharmacol Exp Ther 313:1109–1117 [DOI] [PubMed] [Google Scholar]
- Gayle RB, 3rd, Maliszewski CR, Gimpel SD, Schoenborn MA, Caspary RG, Richards C, Brasel K, Price V, Drosopoulos JH, Islam N, et al. (1998) Inhibition of platelet function by recombinant soluble ecto-ADPase/CD39. J Clin Invest 101:1851–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Quarti-Trevano F, Dell'oro R. (2009) Sympathetic activation in congestive heart failure: evidence, consequences and therapeutic implications. Curr Vasc Pharmacol 7:137–145 [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 73:2424–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan W, Jama A, Donohue T, Wernli G, Onyszchuk G, Al-Hafez B, Bilgen M, Smith PG. (2006) Sympathetic hyperinnervation and inflammatory cell NGF synthesis following myocardial infarction in rats. Brain Res 1124:142–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Khakh BS. (2009) ATP-gated P2X cation-channels. Neuropharmacology 56:208–215 [DOI] [PubMed] [Google Scholar]
- Joseph SM, Pifer MA, Przybylski RJ, Dubyak GR. (2004) Methylene ATP analogs as modulators of extracellular ATP metabolism and accumulation. Br J Pharmacol 142:1002–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius S, Nesbitt S. (1996) Sympathetic overactivity in hypertension. A moving target. Am J Hypertens 9:113S–120S [DOI] [PubMed] [Google Scholar]
- Kittel A, Kiss AL, Müllner N, Matkó I, Sperlágh B. (2005) Expression of NTPDase1 and caveolins in human cardiovascular disease. Histochem Cell Biol 124:51–59 [DOI] [PubMed] [Google Scholar]
- Köhler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Müller CE, Eltzschig HK. (2007) CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation 116:1784–1794 [DOI] [PubMed] [Google Scholar]
- Koyama M, Seyedi N, Fung-Leung WP, Lovenberg TW, Levi R. (2003) Norepinephrine release from the ischemic heart is greatly enhanced in mice lacking histamine H3 receptors. Mol Pharmacol 63:378–382 [DOI] [PubMed] [Google Scholar]
- Kübler W, Strasser RH. (1994) Signal transduction in myocardial ischaemia. Eur Heart J 15:437–445 [DOI] [PubMed] [Google Scholar]
- Lechner SG, Dorostkar MM, Mayer M, Edelbauer H, Pankevych H, Boehm S. (2004) Autoinhibition of transmitter release from PC12 cells and sympathetic neurons through a P2Y receptor-mediated inhibition of voltage-gated Ca2+ channels. Eur J Neurosci 20:2917–2928 [DOI] [PubMed] [Google Scholar]
- Lindholm LH, Carlberg B, Samuelsson O. (2005) Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 366:1545–1553 [DOI] [PubMed] [Google Scholar]
- Machida T, Heerdt PM, Reid AC, Schäfer U, Silver RB, Broekman MJ, Marcus AJ, Levi R. (2005) Ectonucleoside triphosphate diphosphohydrolase 1/CD39, localized in neurons of human and porcine heart, modulates ATP-induced norepinephrine exocytosis. J Pharmacol Exp Ther 313:570–577 [DOI] [PubMed] [Google Scholar]
- Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Alyonycheva TN, Safier LB, Hajjar KA, Posnett DN, Schoenborn MA, Schooley KA, et al. (1997) The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest 99:1351–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Pinsky DJ, Sesti C, Levi R. (2003) Heterologous cell-cell interactions: thromboregulation, cerebroprotection and cardioprotection by CD39 (NTPDase-1). J Thromb Haemost 1:2497–2509 [DOI] [PubMed] [Google Scholar]
- Morrey C, Estephan R, Abbott GW, Levi R. (2008) Cardioprotective effect of histamine H3-receptor activation: pivotal role of Gβγ-dependent inhibition of voltage-operated Ca2+ channels. J Pharmacol Exp Ther 326:871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskvina E, Unterberger U, Boehm S. (2003) Activity-dependent autocrine-paracrine activation of neuronal P2Y receptors. J Neurosci 23:7479–7488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067 [DOI] [PubMed] [Google Scholar]
- Petersson MJ, Rundqvist B, Johansson M, Eisenhofer G, Lambert G, Herlitz H, Jensen G, Friberg P. (2002) Increased cardiac sympathetic drive in renovascular hypertension. J Hypertens 20:1181–1187 [DOI] [PubMed] [Google Scholar]
- Pingle SC, Jajoo S, Mukherjea D, Sniderhan LF, Jhaveri KA, Marcuzzi A, Rybak LP, Maggirwar SB, Ramkumar V. (2007) Activation of the adenosine A1 receptor inhibits HIV-1 tat-induced apoptosis by reducing nuclear factor-κB activation and inducible nitric-oxide synthase. Mol Pharmacol 72:856–867 [DOI] [PubMed] [Google Scholar]
- Schaefer U, Machida T, Broekman MJ, Marcus AJ, Levi R. (2007) Targeted deletion of ectonucleoside triphosphate diphosphohydrolase 1/CD39 leads to desensitization of pre- and postsynaptic purinergic P2 receptors. J Pharmacol Exp Ther 322:1269–1277 [DOI] [PubMed] [Google Scholar]
- Selwyn AP, Braunwald E. (2001) Ischemic heart disease, in Harrison's Principles of Internal Medicine (Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL. eds) pp 1399–1410, McGraw-Hill, New York [Google Scholar]
- Sesti C, Broekman MJ, Drosopoulos JH, Islam N, Marcus AJ, Levi R. (2002) EctoNucleotidase in cardiac sympathetic nerve endings modulates ATP-mediated feedback of norepinephrine release. J Pharmacol Exp Ther 300:605–611 [DOI] [PubMed] [Google Scholar]
- Sesti C, Koyama M, Broekman MJ, Marcus AJ, Levi R. (2003) Ectonucleotidase in sympathetic nerve endings modulates ATP and norepinephrine exocytosis in myocardial ischemia. J Pharmacol Exp Ther 306:238–244 [DOI] [PubMed] [Google Scholar]
- Seyedi N, Mackins CJ, Machida T, Reid AC, Silver RB, Levi R. (2005) Histamine H3-receptor-induced attenuation of norepinephrine exocytosis: a decreased protein kinase A activity mediates a reduction in intracellular calcium. J Pharmacol Exp Ther 312:272–280 [DOI] [PubMed] [Google Scholar]
- Taupenot L. (2007) Analysis of regulated secretion using PC12 cells, in Current Protocols in Cell Biology (Bonifacino JS, Dasso M, Harford JB, Lippincott-Schwartz JB, Yamada KM. eds) chapter 15, unit 15.12, Wiley, New York: [DOI] [PubMed] [Google Scholar]
- Tiscornia G, Singer O, Verma IM. (2006) Production and purification of lentiviral vectors. Nat Protoc 1:241–245 [DOI] [PubMed] [Google Scholar]
- Vassort G. (2001) Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev 81:767–806 [DOI] [PubMed] [Google Scholar]
- Vollmayer P, Koch M, Braun N, Heine P, Servos J, Israr E, Kegel B, Zimmermann H. (2001) Multiple ecto-nucleotidases in PC12 cells: identification and cellular distribution after heterologous expression. J Neurochem 78:1019–1028 [DOI] [PubMed] [Google Scholar]
- von Kügelgen I. (2006) Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110:415–432 [DOI] [PubMed] [Google Scholar]
- Wang TF, Ou Y, Guidotti G. (1998) The transmembrane domains of ectoapyrase (CD39) affect its enzymatic activity and quaternary structure. J Biol Chem 273:24814–24821 [DOI] [PubMed] [Google Scholar]
- Werner P, Seward EP, Buell GN, North RA. (1996) Domains of P2X receptors involved in desensitization. Proc Natl Acad Sci USA 93:15485–15490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall DP, Todorov LD, Mihaylova-Todorova ST. (2002) ATP as a cotransmitter in sympathetic nerves and its inactivation by releasable enzymes. J Pharmacol Exp Ther 303:439–444 [DOI] [PubMed] [Google Scholar]
- Yu JY, DeRuiter SL, Turner DL. (2002) RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci USA 99:6047–6052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicha S, Tsuji Y, Shiroshita-Takeshita A, Nattel S. (2006) Beta-blockers as antiarrhythmic agents. Handb Exp Pharmacol 171:235–266 [PubMed] [Google Scholar]
- Zimmermann H, Braun N. (1999) Ecto-nucleotidases–molecular structures, catalytic properties, and functional roles in the nervous system. Prog Brain Res 120:371–385 [PubMed] [Google Scholar]