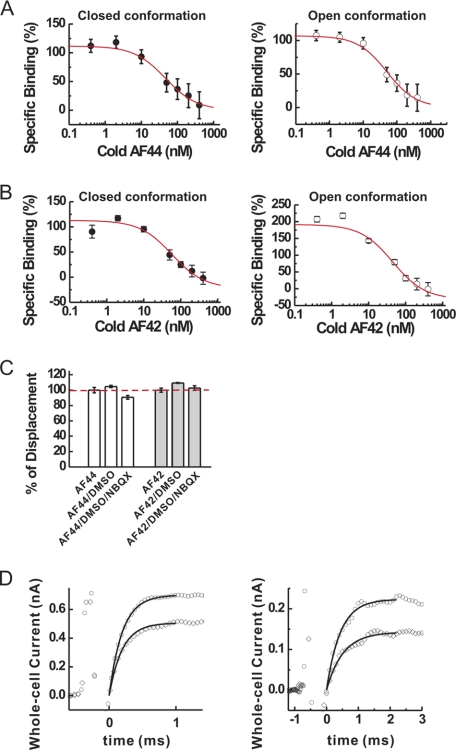
FIGURE 5.
Characterization of the mechanism of inhibition of AF44/AF42 by binding and kinetic measurements. A, by homologous competition binding assay of AF44 (cold and hot) to GluA2Qflip receptor, the binding constant, Kd, was calculated based on triplicate data sets, using Equation 1, to be 44 ± 18 nm for the unliganded, closed-channel conformation (left panel) and 48 ± 13 nm for the open-channel conformation (right panel) of GluA2Qflip, respectively. B, likewise, Kd for AF42 was found to be 57 ± 21 nm for the closed-channel conformation (left panel) and 44 ± 11 nm for the open-channel (right panel) conformation of GluA2Qflip, respectively. C, percentage of displacement of the binding of each aptamer in the presence of NBQX. Binding of hot AF44 or hot AF42 without DMSO and NBQX was set to be 100% (dashed line). As a control, we tested the effect of 8% DMSO on the binding of AF44 and AF42 to GluA2Qflip; this was the same amount of DMSO used to dissolve NBQX. Triplicate data sets for binding were collected, and the average value is displayed. D, the laser-pulse photolysis measurement of the effect of AF44/AF42 on the channel-opening rate of GluA2Qflip. At 300 μm photolytically released glutamate concentration (left panel), kobs was 4975 ± 173 s−1 for the control (open circles), but was 4273 ± 146 s−1 in the presence of 1 μm AF44/AF42 (open diamonds). The first order rate constants were calculated as the best fit (see the solid line in both traces) by Equation 3. The current amplitude in the absence and presence of aptamer was 0.70 and 0.51 nA, respectively. At 100 μm photolytically released glutamate concentration, kobs, which reflected kcl, was similarly estimated to be 2297 ± 36 s−1, and the current amplitude was 0.23 nA in the absence of AF44/AF42 (open circles). In the presence of 1 μm AF44/AF42 (open diamonds), kobs was found to be 2223 ± 25 s−1, and the current amplitude was 0.15 nA. The initial spikes prior to the current rise were discharge signals from the laser flash.