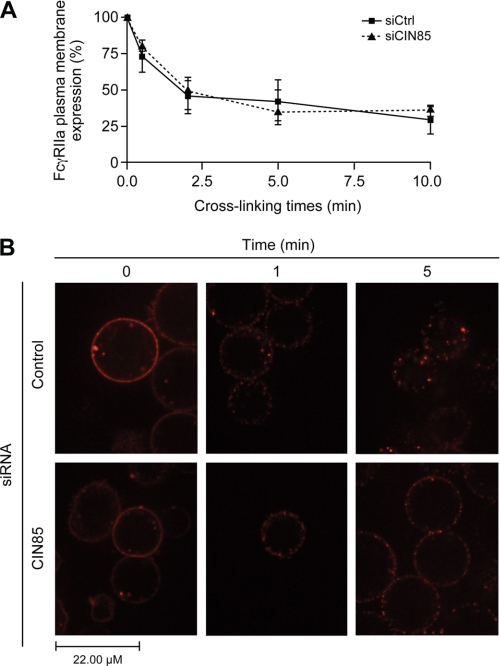
FIGURE 4.
CIN85 is involved in the late steps of FcγRIIa internalization. Bt2cAMP-differentiated PLB-985 cells were transfected with a negative control siRNA or an siRNA against CIN85, as described under “Experimental Procedures. A, the membrane expression of FcγRIIa was monitored by flow cytometry. Transfected cells were stimulated for the indicated times by cross-linking FcγRIIa with a goat anti-mouse F(ab′)2 anti- F(ab′)2 (25 μg/ml) at 37 °C. The stimulations were terminated by transferring the tubes to an ice bath, followed by centrifugation at 400 × g for 2 min at 4 °C. The cell pellets were washed in cold HBSS containing 0.005% BSA and incubated with FITC-labeled goat anti-mouse Fcγ specific IgG (diluted 1:100 in HBSS/BSA) for 30 min on ice. Cells were then washed twice in HBSS/BSA and analyzed by flow cytometry. IgG2b was used as an isotype-matched negative control. The data shown are the quantification of the mean fluorescence intensity of three independent experiments where FcγRIIa levels were normalized to samples without IV.3 cross-linking (100% expression level). B, transfected PLB-985 (107/ml) were incubated with phycoerythrin-conjugated IV.3 (1 μg/ml) for 10 min, washed and plated as described under “Experimental Procedures.” Anti-mouse F(ab′)2 was added in the environment chamber, and cells were visualized live by confocal microscopy for the indicated times. For each condition, at least 25 cells were analyzed. These pictures are representative of three independent cross-linking experiments. Error bars, S.E.