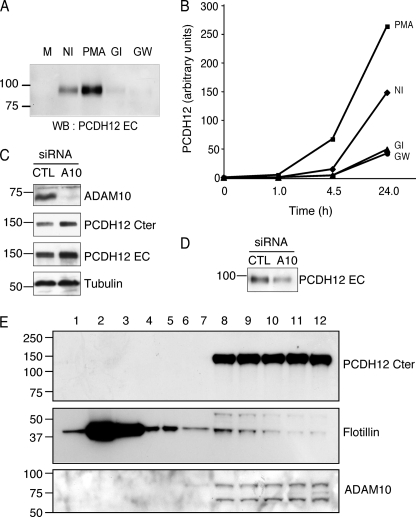
FIGURE 5.
Reduction of PCDH12 shedding after ADAM10 inhibition. A and B, CHO-PCDH12 were treated with either Me2SO (NI), PMA 0.1 μg/ml, GI254923 5 μm (GI), or GW280264 5 μm (GW). Aliquots of cell supernatant were removed at different time points. A, analysis of cell supernatants (20 μl) at 24 h post-induction by Western blotting (WB) with anti-PCDH12 EC. M, unconditioned cell medium. B, dot blot analysis of cell supernatants at different time points, incubated with same antibody. The optical densities were measured on low exposure films. The data are representative of three independent experiments. C, CHO-PCDH12 cells were transfected either with siRNAs against ADAM10 (A10) or control (CTL) siRNAs and grown without PMA. The cell extracts were analyzed by Western blot using antibodies against ADAM10 to prove siRNA efficiency, against PCDH12 Cter and EC to examine PCDH12 stability and against tubulin as loading control. D, effect of ADAM10 siRNAs in PCDH12 release in cell supernatants. E, subcellular fractionation of CHO-PCDH12 using sucrose density gradient. Fractions 1–12 (top to bottom) were analyzed by Western blot with PCDH12 Cter, flotillin, and ADAM10 antibodies. Flotillin, a lipid raft protein marker, is mainly located in fractions 2 and 3, whereas PCDH12 is concentrated in the nonlipid raft fractions (fractions 8–12), together with ADAM10.