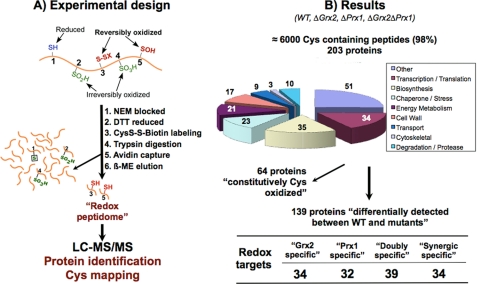
FIGURE 2.
Redox proteomic approach and results. A, free thiols are initially blocked with NEM, and all reversibly oxidized thiols are reduced and subsequently labeled with biotin-HPDP. After tryptic digestion, biotin-labeled peptides are purified and analyzed by MS/MS. This method detects proteins with reversibly oxidized cysteine(s); reduced or irreversibly overoxidized cysteines are not detected. A protein that is not detected in WT but is reversibly oxidized in a mutant is expected to have its cysteine totally reduced in the WT (these proteins are marked with a plus sign in Table 2). A minor percentage of proteins were detected in WT but not in other mutant strain(s), probably due to irreversible overoxidation in the latter, because its antioxidant defenses are further diminished (these proteins are marked with a minus sign in Table 2). B, 203 proteins were consistently identified from the reversibly oxidized Cys-containing peptides. 64 “constitutively Cys-oxidized” proteins were detected in all four strains. A redox target is a protein whose thiol redox state changes differentially between the studied strains. “Grx2-specific” and “Prx1-specific” proteins are those proteins that change as a consequence of the absence of Grx2 and Prx1, respectively. A “doubly specific” protein is a protein specific for both redoxins and is detected with redox changes in both Δgrx2 and Δprx1 single mutants. “Synergic specific” proteins show redox changes only when both redoxins are absent, and are detected only in the double Δgrx2Δprx1 mutant.