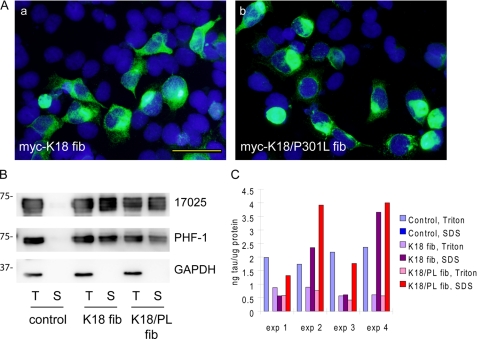
FIGURE 2.
P301L mutation enhances pff-induced aggregation. A, QBI-293 cells were transiently transfected with T40 with P301L mutation (T40/P301L) and transduced with Myc-K18 fibrils (fib) (panel a) or Myc-K18/P301L fibrils (panel b) using BioPORTER reagent. Abundant Tau aggregates were detected using mAb PHF-1 (green) after simultaneous 1% Triton X-100 extraction during fixation to remove soluble proteins. B, sequential extraction was performed on T40/P301L-transfected cells treated with BioPORTER reagent alone (control), with Myc-K18 fibrils (K18 fib), or Myc-K18/P301L fibrils (K18/PL fib). T indicates the cellular fraction recovered in 1% Triton X-100 lysis buffer. S indicates the Triton-insoluble fraction solubilized in 1% SDS lysis buffer. Equal proportions of Triton and SDS fractions were loaded on SDS-polyacrylamide gels. Immunoblotting with polyclonal Tau Ab 17025 and PHF-1 revealed accumulation of abundant Triton-insoluble Tau in fibril transduced cells. GAPDH served as a loading control. C, sandwich Tau ELISA on cell lysates from four independent experiments (exp) confirmed induction of significant amount of Triton-insoluble Tau with fibril transduction, which was always accompanied by a reduction in soluble Tau. Magnification: ×40. Scale bar, 50 μm.