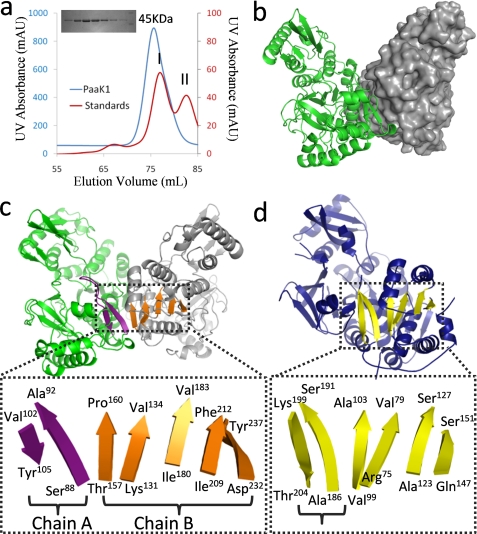
FIGURE 1.
Dimerization of PaaK1 reconstitutes β-sheet organization. a, size exclusion chromatogram with an inset SDS-PAGE gel. PaaK1 (molecular mass, 48 kDa) eluted as an apparent dimer (molecular mass, 82 kDa). Molecular mass (MW) standards conalbumin (I; 75 kDa) and ovalbumin (II; 43 kDa) are shown. b, secondary structure and surface representation of the symmetrical homodimer of PaaK1 with one monomer displayed in green and the second in gray. c, intimate dimerization interactions of PaaK1 create composite β-sheets spanning both monomers. The two-stranded sheet flanking the P-loop (purple) is abutted by the distorted, five-strand sheet (orange) of the second monomer. d, crystal structure of benzoate-CoA ligase (Protein Data Bank code 2V7B; blue) shows the typical familiar tertiary structure with the β-sheet highlighted yellow. The antiparallel strands flanking the P-loop (bracketed) form part of the sheet.