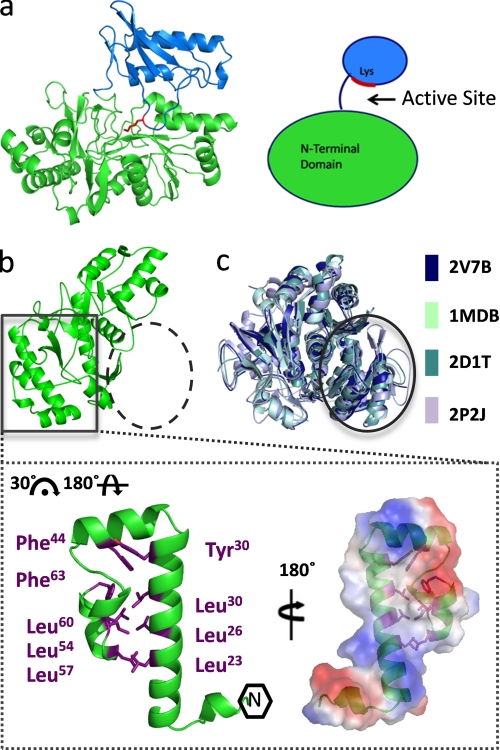
FIGURE 2.
Novel N-terminal microdomain. a, monomer structure of PaaK1 with the larger N-terminal domain in green, the C-terminal domain in blue, and active site conserved lysine shown in red. b, PaaK1 contains a small helical bundle arrangement at the N terminus (boxed) but lacks the typical N-terminal arrangement exhibited by family members (dashed circle). Inset, helical bundle microdomain of PaaK1 is largely stabilized by hydrophobic interactions of Leu and Phe side chains. The electrostatic surface of the microdomain interface predicts a large hydrophobic patch surrounded by charged patches. c, overlays of four homologous family members (Protein Data Bank code 2V7B, benzoate-CoA ligase; Protein Data Bank code 1MDB, 2,4-dihydroxybenzoate AMP ligase; Protein Data Bank code 2D1T, firefly luciferase; Protein Data Bank code 2P2J, acetyl-CoA synthetase) demonstrate the typical α/β-sandwich arrangement at the N terminus (circle).