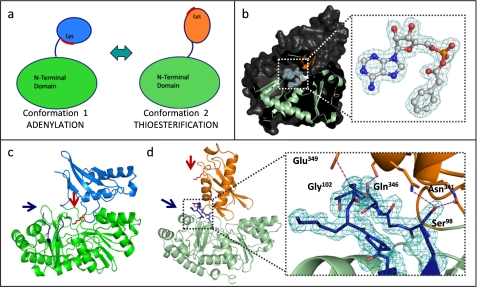
FIGURE 4.
PaaK1 and PaaK2 phenylacetyl-adenylate co-structures captured in alternate conformations. a, schematic of the domain alternation hypothesis. Conformation 1 is utilized for the adenylation part reaction for which the invariant lysine is essential. The C-terminal domain then rotates relative to the N-terminal domain, clearing the lysine from the active site and presenting an alternate face for the thioesterification reaction. b, secondary structure (light green, N-terminal domain; orange, C-terminal domain) and surface (dark gray) representation of PaaK2 captured in conformation 2 with the adenylate intermediate bound within the active site. Inset, 2Fo − Fc electron density mesh (teal) is contoured to 1.0 σ. c, secondary structure representation of PaaK1 phenylacetyl adenylate co-structure in conformation 1 (green, N-terminal domain; light blue, C-terminal domain) with the invariant Lys (red arrow) positioned within the active site. The P-loop of this structure is disordered and not modeled in entirety (blue arrow). d, secondary structure representation of PaaK2 (N-terminal domain, light green; C-terminal domain, orange) in conformation 2. The P-loop is shown in blue, and Lys429 is shown in red. Inset, 2Fo − Fc electron density mesh contoured to 1.0 σ surrounding the P-loop of PaaK2. The P-loop forms four mutually stabilizing hydrogen bonding interactions (purple dashes) with the C-terminal domain.