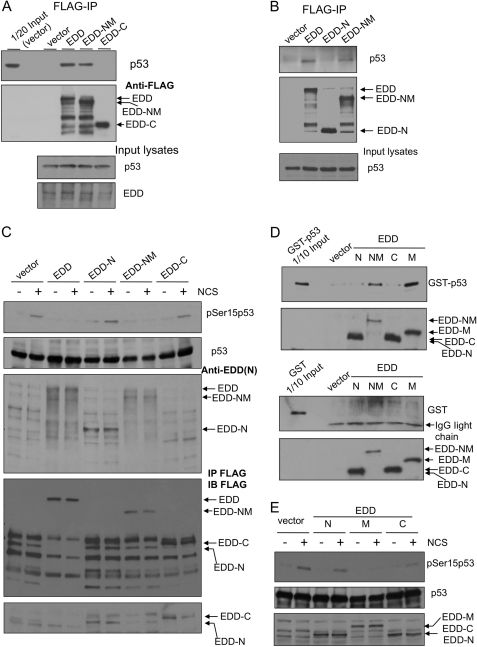
FIGURE 8.
Mapping the interaction region of EDD and identifying its role in inhibiting phosphorylation of p53 at Ser15. A, HEK293 cells were transfected with an empty vector control, FLAG-EDD, or FLAG-tagged truncated mutants: EDD-NM (a.a. 1–1876) or EDD-C (C terminus; a.a. 1876–2799). FLAG-EDD and those mutants were immunoprecipitated (IP) from cell lysates, and the co-immunoprecipitated p53 was detected by immunoblotting. B, HEK293 cells were transfected with an empty vector control, FLAG-EDD, or FLAG-tagged truncated mutants: EDD-N (N terminus; a.a. 1–888) or EDD-NM. Lysates were immunoprecipitated with anti-FLAG beads followed by immunoblotting with p53 antibody. C, HEK293 cells were transfected by electroporation with an empty vector, FLAG-EDD, or FLAG-tagged truncated mutants: EDD-N, EDD-NM, or EDD-C. Two days later, cells were either left untreated or treated by NCS for 1 h. Cell lysates were subjected to Western blotting analysis with the indicated antibodies. The EDD antibody was raised against EDD N terminus and therefore do not recognize EDD-C. To show the expression of EDD-C, we immunoprecipitated these lysates with anti-FLAG beads and then immunoblotted (IB) with a FLAG antibody (the lower two panels). Because vector lanes had nonspecific bands (presumably heavy chain IgG) co-migrating with the EDD-N signal, we reprobed the membrane using a light chain specific secondary antibody (the bottom panel). D, HEK293 cells were transfected with FLAG-EDD or FLAG-tagged truncated mutants. The FLAG-tagged peptides were purified by anti-FLAG beads and then incubated with purified GST or GST-p53 (Fig. 6F), and the bound GST-p53 was pulled down by anti-FLAG beads. E, HEK293 cells were transfected by electroporation with an empty vector, FLAG-EDD, or truncated mutants: EDD-N, EDD-M, or EDD-C. Two days later, cells were either left untreated or treated by NCS for 1 h. Cell lysates were subjected to Western blotting analysis with the indicated antibodies. The FLAG-EDD mutants were identified by FLAG antibody.