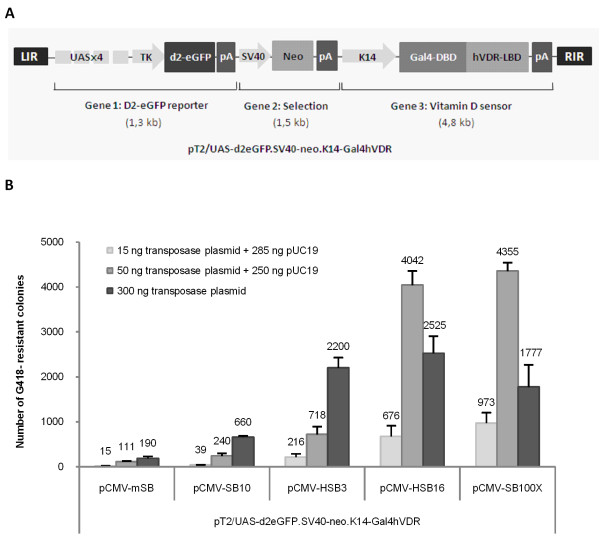
Figure 2.
Effective transposition of the tri-cistronic receptor-sensor SB DNA transposon in HaCaT cells. (A) Schematic representation of the SB transposon-based sensor. Modules of the sensor are flanked by the left and right inverted repeat (LIR and RIR, respectively) of the SB transposon. The 17-bp Upstream Activating Sequence quarto-repeat (UASx4) assists the minimal thymidine kinase (TK) promoter in expressing the destabilized enhanced green fluorescence (d2eGFP) protein flanked by a Simian virus 40 (SV40) polyadenylation sequence. The selection cassette consists of a SV40 promoter driving transcription of the neomycin resistance gene (Neo) tailed by a SV40 polyadenylation sequence. The receptor component consists of the skin-specific keratin 14 (K14) promoter driving expression of the chimeric Gal4 human vitamin D receptor (Gal4hVDR) gene flanked downstream by the K14 polyadenylation sequence. The K14 promoter restricts expression to keratinocytes and derived cell lines or the stratum basale of the skin. (B) Genomic insertion of the genetic sensor catalyzed by a panel of transposases. The transposition assay was carried out with various transposases at different concentrations. HaCaT cells were transfected with 1.7 μg pT2/UAS-d2eGFP.SV40-neo.K14-Gal4hVDR and 300 ng, 50 ng or 15 ng plasmid encoding mSB, SB10, HSB3, HSB16 or SB100X. Additionally, 250 ng or 285 ng pUC19 was included as stuffer to ensure that equal amounts of DNA were used in each transfection. Transposition activity was measured by counting the number of G418-resistant colonies 14 days post-transfection. All experiments were performed in triplicates and are the data shown here as mean values ± standard deviations.