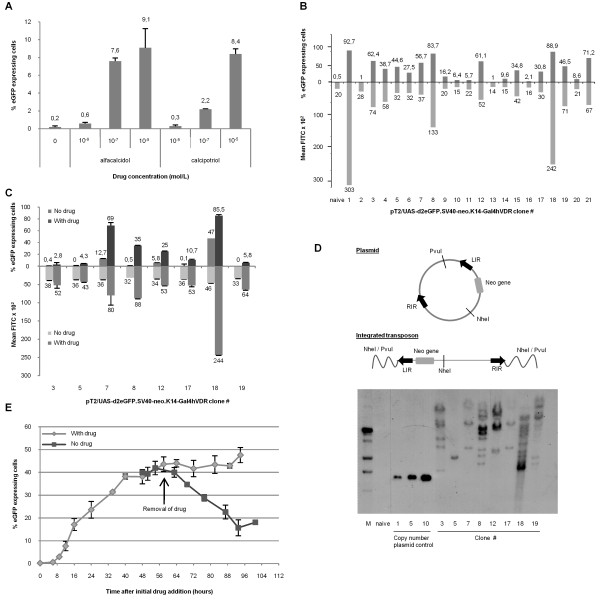
Figure 3.
Generation of stable and highly inducible sensor clones with rapid increase and decline of signal. (A) Sensor induction in HaCaT keratinocytes. HaCaT cells were co-transfected with 1.5 μg pT2/UAS-d2eGFP.SV40-neo.K14-Gal4hVDR and 0.5 μg pCMV-SB100X. Sensor-containing cells were exposed to DMSO-dissolved vitamin D3 analogues at different concentrations for 48 hours and analyzed by flow cytometry. (B) Induction of sensor in individual HaCaT clones. Cells were exposed to DMSO-dissolved 10-5 M alfacalcidol and analyzed by flow cytometry after 2 days. D2eGFP expression levels are shown in the first quadrant and mean FITC intensity in the second quadrant. Registered signal in the FITC channel from un-transfected (naive) HaCaT cells served as reference. (C) Sensor activity in the absence and presence of drug. Eight clones selected among the 21 sensor-containing clones were incubated 48 hours in absence or presence of 10-5 M alfacalcidol and subsequently evaluated by flow cytometry. (D) Southern blotting shows variable integration patterns among the vitamin D3-responsive clones. (E) Kinetics of the sensor upon exposure to vitamin D3 analogues. Cells from clone #8 were initially split in two sets that were grown in parallel. With regular time intervals medium containing 10-5 M alfacalcidol was added to the cells. At 52 hours incubation one set was relieved from the compound and cultured in drug-free media. At the end of the experimental time frame, the cells were analyzed by flow cytometry. The experiments in (A), (B), and (E) were conducted in triplicate, and data are shown as mean values ± standard deviations.