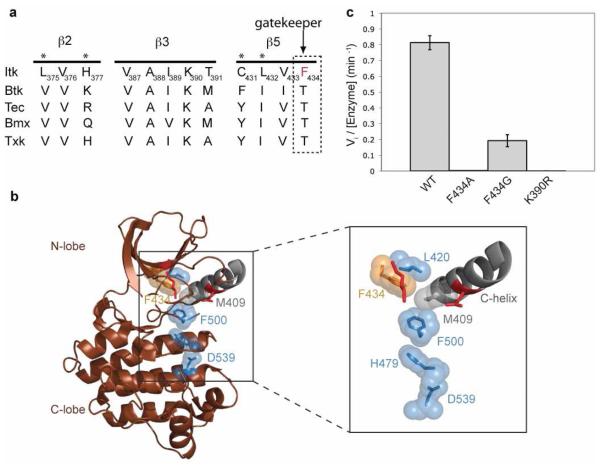
Figure 1. The Itk gatekeeper residue F434 promotes the assembled state of the regulatory spine.
(a) Alignment of the β2, β3 and β5 strands of the N-terminal lobe of the kinase domain of Tec kinases. The gatekeeper residue of Itk is a phenylalanine (F434), all other Tec kinases contain a threonine at the gatekeeper position (indicated with arrow). Asterisks above the sequences indicate targets for the second-site mutagenesis. (b) The crystal structure of the isolated Itk kinase domain in the absence of the SH2-kinase linker (brown cartoon, PDB ID 1SNX) is shown with the disassembled regulatory spine residues: M409 (grey sticks and spheres), L420, H479, F500 and D539 (blue sticks and spheres). The boxed region is expanded to show the Itk gatekeeper F434 (orange sticks and spheres), and the C-helix is labeled. The crucial ion pair (Itk K390 and E405) that is assembled in active kinase structures is shown as red sticks and is not labeled. All structures in this and other figures were generated using PyMOL (33). (c) The gatekeeper residue of Itk (F434) was mutated to either alanine or glycine in the context of full-length Itk and tested for its in vitro kinase activity as described in materials and methods. Mutation of F434 to alanine or glycine reduces the activity of full-length Itk to almost that of the kinase inactive mutant (K390R) of full-length Itk.