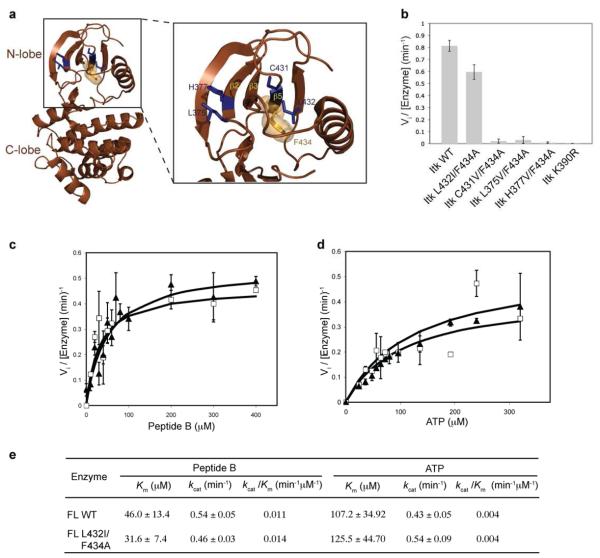
Figure 2. The Itk L432I/F434A double mutation rescues the activity of the Itk gatekeeper F434A mutation.
(a) Location of potential second-site activating mutations within the N-terminal lobe of the Itk kinase domain. The crystal structure of the Itk kinase domain (brown cartoon) with the β2, β3 and β5 strands of the N-terminal lobe are indicated. The location of the four potential activating sites (L375, H377, C431 and L432) are shown as blue sticks and the gatekeeper (F434) residue is shown in orange (sticks and spheres). (b) The L432I mutation rescues the activity of the F434A gatekeeper mutation in Itk. The L375V, H377V, C431V and L432I mutations were made in the context of full-length Itk F434A, and the double mutants were tested for kinase activity as described in materials and methods. The Itk L432I/F434A double mutation recovers the activity of the Itk F434A mutant. (c, d and e) Kinetic parameters of the full-length Itk L432I/F434A double mutant. Substrate (peptide B or ATP) curves of wild-type full-length Itk (FL WT, filled triangles) or Itk L432I/F434A double mutant (FL L432I/F434A, open squares), were fit to the Michaelis-Menten equation using GraphFit to obtain the kinetic parameters reported in (e). The values for full-length wild-type Itk have been reported previously (10).