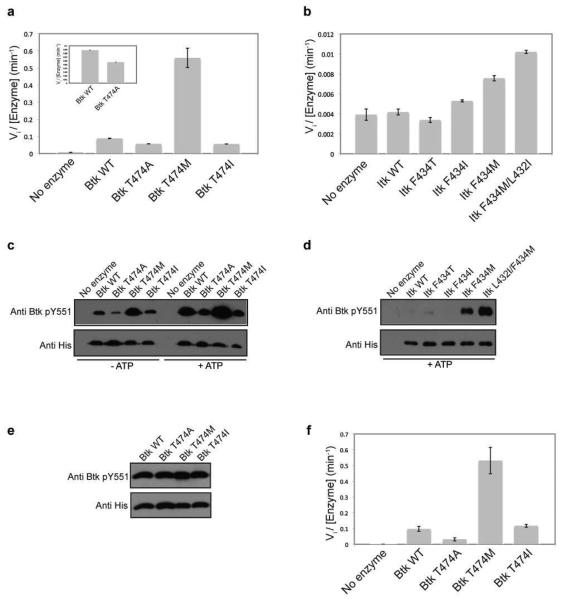
Figure 3. Mutation of the gatekeeper residue to methionine activates Tec kinases in the absence of the N-terminal regulatory domains.
(a and b) The gatekeeper residues of Itk and Btk were mutated to alanine, methionine, isoleucine and threonine in the context of the isolated kinase domains and tested for in vitro kinase activity as described in Experimental Procedures. Mutation of the Itk and Btk gatekeeper residues, F434 and T474 respectively, to methionine activates the isolated kinase domains of Itk and Btk. The inset in (a) shows the Btk T474A mutant is approximately half as active as wild type Btk kinase domain. (c and d) Activity of the Tec kinase gatekeeper mutations correlates with the level of phosphorylation on the activation loop of the kinase. Purified 250 nM kinase domain of Btk wild-type, T474A, T474I or T474M enzymes or Itk wild-type, F434T, F434I, F434M or L432I/F434M enzymes were incubated in a kinase assay buffer at RT for one hour without or with ATP, separated by SDS-PAGE, transferred onto polyvinylidene difluoride (PVDF) membrane, and probed with either an anti-Btk pY551 (also used to detect Itk pY511) or anti-His antibody. The exposure time for the Itk panel is ten times longer than that for the Btk panel. (e) Normalization of phosphorylation level on Btk Y551. Purified 250 nM kinase domain of Btk wild-type, T474A, T474I or T474M enzymes were pre-incubated with ATP in a kinase assay buffer as described in Experimental Procedures. The Btk enzymes were then probed with an anti-Btk pY551 and anti-His antibody as before. (f) The Btk kinase domain enzymes in (e) that were normalized for phosphorylation on Btk Y551 were tested for in vitro kinase activity as before.