Abstract
Microscopy remains the gold standard for malaria diagnosis. However, quality microscopy services are severely lacking in most African countries. To improve capacity for malaria microscopy in Uganda, a 3-day refresher training program was conducted in four districts. Training impact was measured through a written examination and evaluation of the quality of blood-slide preparation and accuracy of field microscopy. A total of 184 of 192 (96%) identified laboratory personnel participated in the training. Average test scores improved from 41% to 75% (P < 0.001). A total of 1,079 and 1,190 routinely made thick blood smears were collected before and after the training, respectively. Sensitivity improved from 84% to 95% (P < 0.001), and specificity improved from 87% to 97% (P < 0.001). The proportion of well-prepared blood smears improved from 6% to 75% (P < 0.001). Supplemental training can have a significant impact on the knowledge of staff, accuracy of microscopy, and quality of blood-slide preparation.
Introduction
Prompt and accurate diagnosis of malaria is one of the cornerstones of effective case management. With the adoption of highly effective but relatively expensive artemisinin-based combination therapy (ACT), there is an increasing need for restricted and better targeted treatment of malaria. Indeed, recently published guidelines from the World Health Organization recommend a parasitological confirmation of diagnosis in all patients suspected of having malaria before treating.1 High-quality malaria diagnosis is important, because it improves patient care in parasite-positive patients, allows for the identification of parasite-negative patients in whom another diagnosis should be sought, reduces the use of unnecessary antimalarials, improves the accuracy of malaria surveillance, and provides confirmation of treatment failures.1
The two methods routinely used for the parasitological diagnosis of malaria are light microscopy and rapid diagnostic tests (RDTs). Microscopy is currently the gold standard and has the advantages of lower cost and the abilities to characterize species, quantify parasite density, and assess response to antimalarial therapy.2,3 Development and maintenance of effective microscopy requires an organized health system, the provision of supplies and reagents, the presence and maintenance of satisfactory microscopes, an adequate workplace environment, and perhaps most importantly, well-trained personnel who are able to accurately prepare and read blood slides.2
There have been relatively few reports of training programs to improve the quality and accuracy of malaria microscopy in Africa, and these evaluations have shown mixed results.4–7 In 2009, the Uganda Malaria Surveillance Project (UMSP) in partnership with the Uganda National Malaria Control Program embarked on a training program for laboratory personnel in malaria microscopy in selected districts with support from the Presidential Malaria Initiative (PMI). Here, we describe the impact of the training program in four districts.
Methods
Study sites.
The training intervention was implemented in four districts of Uganda (Tororo, Masindi, Kanungu, and Kabale) between February and August of 2009. The districts were selected based on Ministry of Health (MOH) priorities, and they represent the diversity of malaria transmission intensity in Uganda, including two holoendemic sites, one mesoendemic site, and one site with unstable transmission (Figure 1).8 A baseline training-needs assessment was done for each district to collect information on the status of laboratory facilities, staffing level, use of laboratory services, and other challenges faced in the laboratories. All facilities that offered malaria microscopy diagnostic services were identified and visited, including private clinics, government health centers, and private and public hospitals. A standard checklist was used in all districts to collect information on laboratory staffing, basic equipment and their status, stock of laboratory supplies, adequacy of working space, and presence of standard operational procedures and other guidelines for malaria laboratory diagnosis.
Figure 1.
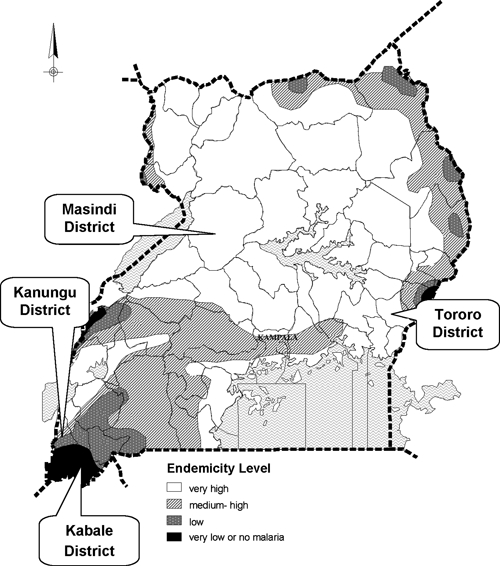
Map of malaria endemicity in Uganda in the four districts where training was conducted.
Refresher training course in malaria microscopy.
The aim of the refresher training course was to improve the quality and accuracy of malaria diagnostics by existing laboratory staff working in facilities with access to microscopy. The curriculum and training materials were developed by a team of laboratory experts from UMSP, MOH, and Central Public Health Laboratories as well as district laboratory focal persons. Key stakeholders were involved to ensure consensus and cohesion with national case management guidelines. The result of this exercise was the development of a 3-day comprehensive in-service training course emphasizing practical diagnosis of malaria in a routine clinical setting. Details of the training curriculum and teaching materials are available at http://www.muucsf.org/publications/training_materials.html.
The course targeted four categories of laboratory personnel typically working in health facilities in Uganda: laboratory technicians, laboratory technologists, laboratory assistants, and microscopists. The course was designed to be conducted over 3 days, with approximately 75% of the time dedicated to participatory methods, including group discussions, role playing, and hands-on experience. Training topics included good laboratory practice, quality control/quality assurance, maintenance of equipment, preparation of reagents, preparation and staining of thin and thick blood smears, examination of blood smears for identification of parasites/speciation/parasite density, standard methods for reporting results, and record keeping. Training was conducted by a team of experienced laboratory technicians who traveled to each district to conduct the course at a selected central health facility. To minimize disruption of patient services, the laboratory staff at each health facility were divided into two groups and trained in two back to back 3-day sessions.
Assessing the impact of training.
Three methods were used to assess the impact of training: (1) pre- and post-testing administered at the beginning and end of the training course, (2) quality control reading of blood smears from randomly selected participating laboratories before training and approximately 1 month after training, and (3) quality control assessment of blood-slide preparation. The pre- and post-testing consisted of a standardized written examination including multiple-choice questions and the reading of well-characterized slides and digital pictures to assess knowledge of key concepts and facts.
For quality control reading, our goal was to collect 50–100 thick blood smears from consecutive patients referred to the laboratory both before and after training from five health facilities in Tororo, four health facilities in Masindi and Kanungu, and three health facilities in Kabale. Thick blood smears were stained with 10% Giemsa for 10 minutes and read as positive or negative for malaria parasites by health facility staff as part of their routine practice. Thick blood smears were mounted and subsequently sent to a central laboratory in Kampala, Uganda, to be read by expert microscopists who were blinded to field results. Asexual parasitemia of any level was reported as a positive smear, and smears were considered negative if the examination of 100 high-power fields did not show asexual parasites. Discrepant field and first expert reading were reviewed by a second expert microscopist. Results were considered final if the first and second expert readings agreed on parasites presence and species. If the first and second expert readings were discordant, the tie was broken by a third expert reader.
The quality of the blood smears was further graded as poor, fair, or good by consideration of the size, thickness, and staining of the smear. A good smear was defined as being at least 10 mm away from the edge of the slide, round in shape with a diameter of approximately 10 mm, and a density that fine print could just be read through it; additionally, a good smear has all of the red blood cells lysed, and the malaria parasites should be well-exposed with a bluish pink coloration. A poorly made blood smear was defined as being too big (diameter greater than 10 mm), very thick (fine print could not be read through it), or poorly stained (red blood cells were not lysed and parasites had a green, red, or blue color). A fair slide was defined as being well-made (right size and thickness) but moderately stained (red blood cells were partially lysed and parasites were lightly stained).
Data management and statistical analysis.
Results of the written examinations, quality of blood-slide preparation, and quality control microscopy readings were entered into a centralized database using Access (Microsoft Corporation, Redmond, WA). Statistical analysis was done using Stata version 10 (Stata, College Station, TX). The accuracy of quality control blood-smear reading was expressed in terms of the sensitivity and specificity of field microscopy, with expert microscopy as the gold standard. Proportions were compared using the χ2 test. A P value < 0.05 was considered statistically significant.
Results
Assessment of laboratory services.
All functional health facilities in the four districts were identified and assessed for the presence of malaria microscopy services: Tororo district had 63 functional health facilities, 15 (24%) of which offered microscopy, Masindi district had 51 functional health facilities, 30 (59%) of which offered microscopy, Kanungu district had 46 functional health facilities, 16 (35%) of which offered microscopy, and Kabale district had 84 functional health facilities, 36 (43%) of which offered microscopy. A baseline training-needs assessment was conducted at all facilities that offered malaria microscopy to collect information on the status of laboratory facilities and staffing levels. All of the laboratory facilities were in fairly good physical conditions, but only 76 (78%) had sufficient space, 69 (71%) had electricity, and 44 (45%) had running water; 65 facilities had only one functional microscope, 25 had two functional microscopes, and 7 had three or more functional microscopes. A total of 89 (92%) health facilities had sufficient reagents to perform malaria diagnosis, including stock solutions of the essential reagents. Laboratory record books were available at 90 (93%) facilities, but only 44 (45%) had standard operating procedures or guidelines for malaria diagnosis. Laboratory personnel from 44 (45%) laboratories reported that they were performing quality control and quality assurance for malaria diagnosis; however, there was no evidence showing the implementation of these measures.
Health facilities offering microscopy in the four districts had a total of 192 laboratory staff capable of performing malaria microscopy: 11 (6%) laboratory technologists, 27 (14%) laboratory technicians, 102 (53%) laboratory assistants, and 52 (27%) microscopists. The number of laboratory staff working in facilities that offered microscopy varied from 26 in Kanungu district to 73 in Kabale district (Table 1).
Table 1.
Characteristics of trainees and pre- and post-training didactic test scores
| District | Number identified | Number trained | Type of health facility | N (%) | Type of position | N (%) | Average didactic test score (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|
| Pre-training | Post-training | P value | |||||||
| Tororo | 44 | 41 (93%) | Private clinic | 5 (12%) | Microscopist | 10 (24%) | 41% (36–46%) | 62% (59–65%) | < 0.001 |
| Government health center | 21 (51%) | Laboratory assistant | 19 (46%) | ||||||
| Private hospital | 6 (15%) | Laboratory technician | 11 (24%) | ||||||
| Public hospital | 9 (22%) | Laboratory technologist | 1 (2%) | ||||||
| Masindi | 49 | 47 (96%) | Private clinic | 14 (30%) | Microscopist | 21 (45%) | 37% (32–41%) | 81% (78–83%) | < 0.001 |
| Government health center | 23 (49%) | Laboratory assistant | 21 (45%) | ||||||
| Private hospital | 0 | Laboratory technician | 5 (11%) | ||||||
| Public hospital | 10 (21%) | Laboratory technologist | 0 | ||||||
| Kanungu | 26 | 26 (100%) | Private clinic | 1 (4%) | Microscopist | 7 (27%) | 37% (30–43%) | 77% (73–81%) | < 0.001 |
| Government health center | 16 (62%) | Laboratory assistant | 16 (62%) | ||||||
| Private hospital | 0 | Laboratory technician | 2 (8%) | ||||||
| Public hospital | 9 (35%) | Laboratory technologist | 1 (4%) | ||||||
| Kabale | 73 | 70 (96%) | Private clinic | 21 (30%) | Microscopist | 11 (16%) | 45% (42–49%) | 78% (76–80%) | < 0.001 |
| Government health center | 37 (53%) | Laboratory assistant | 43 (61%) | ||||||
| Private hospital | 2 (3%) | Laboratory technician | 7 (10%) | ||||||
| Public hospital | 10 (14%) | Laboratory technologist | 9 (12%) | ||||||
| All districts | 192 | 184 (96%) | Private clinic | 41 (22%) | Microscopist | 49 (26%) | 40% (37–45%) | 75% (62–81%) | < 0.001 |
| Government health center | 97 (53%) | Laboratory assistant | 99 (54%) | ||||||
| Private hospital | 8 (4%) | Laboratory technician | 25 (14%) | ||||||
| Public hospital | 38 (21%) | Laboratory technologist | 11 (6%) | ||||||
CI = confidence interval.
Quantitative impact of the laboratory refresher training course.
A total of 184 of 192 (96%) identified laboratory personnel participated in the refresher training course. Characteristics of the trainees and pre- and post-training didactic test scores are shown in Table 1. Pre-training didactic test scores were generally low, with an average score of 41% (range = 37–45% in the four districts). After training, didactic test scores improved significantly, with an average score of 75% (range = 62–81% in the four districts) (Table 1).
A total of 1,079 thick blood smears were collected at selected health facilities before the training course, and 1,190 blood smears were collected approximately 1 month after the training course (Table 2). The sensitivity of field microscopy (correctly identifying the presence of malaria parasites) was fairly high before training in three of four districts, with sensitivity ranging from 84% to 88%. Kabale district had the lowest sensitivity (69%) before training. After training, sensitivity ranged from 93% to 96%, with significant improvement at all the districts with the exception of Kanungu, which had the highest pre-training sensitivity. Overall, sensitivity improved from 84% to 95% (P < 0.001) after training (Table 2). The specificity of field microscopy (correctly reading a negative blood smear) ranged from 80% to 94% before training. After training, specificity ranged from 92% to 100%, with significant improvements in all four districts. Overall, specificity improved from 87% to 97% (P < 0.001) after training (Table 2).
Table 2.
Diagnostic accuracy of field microscopy pre- and post-training
| District | Health facility | Pre-training | Post-training | Sensitivity pre- and post-training | Specificity pre- and post-training | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N* | SPR† | N* | SPR† | Pre | Post | P value | Pre | Post | P value | ||
| Tororo | Mifumi Health Center III | 100 | 43.0% | 43 | 46.5% | 90.7% | 90.0% | 0.93 | 70.2% | 87.0% | 0.12 |
| St. Anthony's Hospital | 75 | 29.3% | 71 | 23.9% | 81.8% | 88.2% | 0.58 | 84.9% | 90.7% | 0.36 | |
| Nagongera Heath Center IV | 99 | 73.7% | 100 | 51.0% | 79.5% | 100% | < 0.001 | 80.8% | 89.8% | 0.27 | |
| Mulanda Health Center IV | 50 | 56.0% | 62 | 56.5% | 89.3% | 91.4% | 0.77 | 86.4% | 96.3% | 0.21 | |
| Rubongi Military Hospital | 32 | 15.6% | 44 | 43.2% | 80.0% | 100% | 0.05 | 96.3% | 96.0% | 0.96 | |
| All facilities in district | 356 | 48.0% | 320 | 44.4% | 84.2% | 95.1% | 0.002 | 81.6% | 91.6% | 0.006 | |
| Masindi | Kiryandongo Hospital | 82 | 72.0% | 51 | 15.7% | 88.1% | 87.5% | 0.96 | 65.2% | 100% | < 0.001 |
| Masindi Hospital | 28 | 3.6% | 52 | 26.9% | 100% | 92.9% | 0.78 | 88.9% | 97.4% | 0.16 | |
| Masindi Military Hospital | 50 | 40.0% | 99 | 37.4% | 90.0% | 100% | 0.05 | 70.0% | 93.6% | 0.002 | |
| Mutunda Heath Center III | 100 | 32.0% | 38 | 23.7% | 81.3% | 88.9% | 0.59 | 86.8% | 100% | 0.04 | |
| All facilities in district | 260 | 43.1% | 240 | 28.3% | 86.6% | 95.6% | 0.05 | 80.4% | 97.1% | < 0.001 | |
| Kanungu | Kayonza Health Center III | 64 | 51.6% | 76 | 61.8% | 97.0% | 100% | 0.23 | 77.4% | 89.7% | 0.20 |
| Kihihi Heath Center IV | 100 | 28.0% | 97 | 14.4% | 85.7% | 85.7% | 1.0 | 91.7% | 98.8% | 0.03 | |
| Bwindi Hospital | 9 | 0% | 100 | 6.0% | None | 66.7% | – | 100% | 100% | 1.0 | |
| Kambuga Hospital | 100 | 7.0% | 100 | 7.0% | 57.1% | 85.7% | 0.23 | 100% | 100% | 1.0 | |
| All facilities in district | 273 | 24.9% | 373 | 19.8% | 88.2% | 93.2% | 0.30 | 93.7% | 98.7% | 0.002 | |
| Kabale | Rugalama Hospital | 50 | 24.0% | 58 | 82.8% | 50.0% | 97.9% | < 0.001 | 100% | 100% | 1.0 |
| Hamurwa Health Center IV | 40 | 40.0% | 100 | 28.0% | 87.5% | 92.9% | 0.55 | 91.7% | 100% | 0.01 | |
| Rubanda Health Center III | 100 | 11.0% | 99 | 18.0% | 63.6% | 94.4% | 0.03 | 86.5% | 100% | < 0.001 | |
| All facilities in district | 190 | 20.5% | 257 | 36.6% | 69.2% | 95.7% | < 0.001 | 90.7% | 100% | < 0.001 | |
| All districts | All health facilities | 1,079 | 36.1% | 1,190 | 31.8% | 84.1% | 95.0% | < 0.001 | 86.9% | 97.0% | < 0.001 |
Number of slides collected in the field.
Slide positivity rate (SPR) is the proportion of slides read as positive based on expert microscopy.
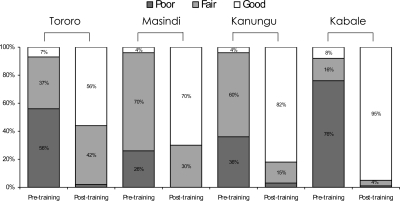
The quality of blood-smear preparations improved dramatically after training. Across the four districts, the range of blood smears classified as good quality ranged from 4% to 8% before training and increased to 56–95% after training (P < 0.001 for all comparisons in the four districts) (Figure 2).
Figure 2.
Quality of blood-slide preparations pre- and post-training at four districts in Uganda (Tororo District: 356 slides pre-training and 320 slides post-training; Masindi District: 260 slides pre-training and 240 slides post-training; Kanungu District: 273 slides pre-training and 373 slides post-training; Kabale District: 190 slides pre-training and 257 slides post-training). For comparisons of slide quality pre- and post-training, P value < 0.001 in all four districts.
Discussion
Here, we described the establishment and impact of a training program in malaria microscopy that was implemented at four districts in Uganda to build local capacity and improve test accuracy. A total of 192 personnel capable of performing malaria microscopy were identified in four districts, which have a combined population of over 1 million people, resulting in approximately 1 microscopist for every 5,000 residents. These numbers highlight the severe lack of laboratory capacity in Uganda given that the incidence of malaria has been estimated to be 478 cases per 1,000 persons per year.9 Most of the laboratory personnel were low-level cadres (microscopists or laboratory assistants) who came from upper-level health facilities and hospitals, characteristics that have been described in other African countries.4
Training led to an improvement in didactic test scores, reflecting an increase in knowledge of key concepts and facts about malaria microscopy. However, documenting increases in knowledge is not the same as documenting improvements in performance.
One of the strengths in the evaluation of this training program was the comparison of diagnostic accuracy before and after training under real world conditions. It is well-recognized that blood-smear readings are highly dependent on the level of experience of the microscopist, techniques used to prepare and stain the blood slide, and quality control standards.10–12 The diagnostic accuracy of field microscopy was surprisingly good before the training program, with an overall sensitivity of 84% and specificity of 87%. However, these findings may have been influenced by the fact that personnel were aware that slides were being collected for evaluation purpose. A high sensitivity is especially important clinically because of the risk of not treating a true case of malaria. A high specificity is important for reducing the use of unnecessary antimalarials and prompting clinicians to look for alternative causes of illness. After training, diagnostic accuracy improved significantly and consistently across most of the health facilities evaluated. Indeed, the post-training sensitivity and specificity of 95% and 97%, respectively, were excellent and higher than have been reported in other malaria diagnostic training programs from Africa.5–7 A similar level of diagnostic accuracy was achieved in Kisumu, Kenya, where a malaria diagnostic center of excellence has been established, providing an intensive long course followed by short-course refresher training.13
One of the main objectives of the training was to improve malaria blood-slide preparation techniques. Indeed, improvement in malaria blood-slide preparation is the first step to improvement in the accuracy of reading malaria blood slides. The poor quality of blood-smear preparation before this training program was dramatically improved after training, with the proportion of well-prepared slides increasing on average from 6% to 75%. In practice, microscopy varies a great deal in different settings because of variable techniques of blood-film preparation, staining, malaria film-reading standards, and level of expertise of the examining microscopists.10,11 Poor blood-film preparation can lead to false-positive results because of the presence of artefacts commonly mistaken for malaria parasites or false-negative results because of inadequate staining, and it increases the time and effort that it takes to read a blood smear.14 The preparation of high-quality blood slides not only requires proper training but also the provision of essential supplies (clean slides, good stain, proper buffer, etc.) and a quality control system to ensure that proper procedures are being followed.
Potential benefits of parasite-based diagnosis depend on accurate results and the proper use of these results in patient management. A prompt and accurate diagnosis is critical for guiding rationale therapeutic decision making for both malaria and non-malaria illnesses.15 Most malaria-endemic countries have now adopted the use of highly effective but expensive ACT for the treatment of malaria.1 Restricted and better directed treatment of malaria is increasingly being advocated to prevent the unnecessary use of ACTs and hence, reduce drug pressure selecting for resistant parasites and frequency of potential adverse drug reactions.16,17 A lack of confidence in accuracy has been cited as a common reason for clinicians ignoring test results, which most commonly manifests in the practice of prescribing antimalarials to patients with negative blood smears.18,19 Improving and documenting the accuracy of microscopy under field conditions is critical for instilling confidence in clinicians so that they can make rational decisions when prescribing antimalarials.
In summary, requirements for laboratory services are growing in malaria-endemic countries where there is a move away from empiric therapy and to parasitological confirmation of the diagnosis. In Uganda, there is a shortage of laboratory personnel, and the quality assurance system is not fully functional. The District Directorate of Health is responsible for maintaining quality laboratory services with national support from the Central Public Health Laboratory. The program described here shows that supplemental training can have a significant impact on the accuracy of malaria microscopy and achieve excellent results in the short term. Efforts are currently underway to expand this refresher training program around the country, and continued supervision and support are essential to ensure sustainability of accurate diagnosis and thereby, appropriate treatment of malaria. These findings should be interpreted in light of the fact that evaluation closely followed training. A long-term monitoring of performance is essential to recommend the appropriate frequency of refresher training.
ACKNOWLEDGMENTS
The authors thank the staff of participating health facilities for their enthusiastic cooperation with the training intervention evaluated in this study. We are grateful to the Uganda Malaria Control Program and other stakeholders who participated in development and approval of the training program. The authors thank the trainers, Sarah Nayunja and Osuwat Lawrence Obado, for their commitment and hard work, which has made this training a success.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Financial support: This study received financial support from the President's Malaria Initiative through a cooperative agreement with the Centers for Disease Control and Prevention (U50/CCU925122).
Authors' addresses: Moses Kiggundu, Uganda Malaria Surveillance Program, Mulago Hospital Complex, Kampala, Uganda, E-mail: mkiggundu@muucsf.org. Samuel L. Nsobya, Uganda Malaria Surveillance Program, Mulago Hospital Complex, Kampala, Uganda, E-mail: samnsobya@yahoo.co.uk. Moses R. Kamya, Makerere University, University of California San Francisco, Malaria Research Collaboration, Kampala, Uganda, E-mail: mkamya@nfocom.co.ug. Scott Filler, Malaria Branch, Division of Parasitic Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: SFiller@cdc.gov. Sussan Nasr, Centers for Disease Control and Prevention, President's Malaria Initiative, Kampala, Uganda, E-mail: icz1@cdc.gov. Grant Dorsey, University of California, San Francisco, CA, E-mail: gdorsey@medsfgh.ucsf.edu. Adoke Yeka, Uganda Malaria Surveillance Program, Mulago Hospital Complex, Kampala, Uganda, E-mail: yadoke@muucsf.org.
References
- 1.World Health Organization . Guidelines for the Treatment of Malaria. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 2.Bell D, Perkins MD. Making malaria testing relevant: beyond test purchase. Trans R Soc Trop Med Hyg. 2008;102:1064–1066. doi: 10.1016/j.trstmh.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Molyneux M, Fox R. Diagnosis and treatment of malaria in Britain. BMJ. 1993;306:1175–1180. doi: 10.1136/bmj.306.6886.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates I, Bekoe V, Asamoa-Adu A. Improving the accuracy of malaria-related laboratory tests in Ghana. Malar J. 2004;3:38. doi: 10.1186/1475-2875-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngasala B, Mubi M, Warsame M, Petzold MG, Massele AY, Gustafsson LL, Tomson G, Premji Z, Bjorkman A. Impact of training in clinical and microscopy diagnosis of childhood malaria on antimalarial drug prescription and health outcome at primary health care level in Tanzania: a randomized controlled trial. Malar J. 2008;7:199. doi: 10.1186/1475-2875-7-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkinfada F, Aliyu Y, Chavasse C, Bates I. Impact of introducing integrated quality assessment for tuberculosis and malaria microscopy in Kano, Nigeria. J Infect Dev Ctries. 2009;3:20–27. doi: 10.3855/jidc.101. [DOI] [PubMed] [Google Scholar]
- 7.Ssekabira U, Bukirwa H, Hopkins H, Namagembe A, Weaver MR, Sebuyira LM, Quick L, Staedke S, Yeka A, Kiggundu M, Schneider G, McAdam K, Wabwire-Mangen F, Dorsey G. Improved malaria case management after integrated team-based training of health care workers in Uganda. Am J Trop Med Hyg. 2008;79:826–833. [PubMed] [Google Scholar]
- 8.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, D'Alessandro U, Coosemans M. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–225. [PubMed] [Google Scholar]
- 9.World Health Organization . World Malaria Report. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 10.Durrheim DN, Becker PJ, Billinghurst K. Diagnostic disagreement—the lessons learnt from malaria diagnosis in Mpumalanga. S Afr Med J. 1997;87:609–611. [PubMed] [Google Scholar]
- 11.Kachur SP, Nicolas E, Jean-Francois V, Benitez A, Bloland PB, Saint Jean Y, Mount DL, Ruebush TK, 2nd, Nguyen-Dinh P. Prevalence of malaria parasitemia and accuracy of microscopic diagnosis in Haiti, October 1995. Rev Panam Salud Publica. 1998;3:35–39. doi: 10.1590/s1020-49891998000100006. [DOI] [PubMed] [Google Scholar]
- 12.Kilian AH, Metzger WG, Mutschelknauss EJ, Kabagambe G, Langi P, Korte R, von Sonnenburg F. Reliability of malaria microscopy in epidemiological studies: results of quality control. Trop Med Int Health. 2000;5:3–8. doi: 10.1046/j.1365-3156.2000.00509.x. [DOI] [PubMed] [Google Scholar]
- 13.Ohrt C, Obare P, Nanakorn A, Adhiambo C, Awuondo K, O'Meara WP, Remich S, Martin K, Cook E, Chretien JP, Lucas C, Osoga J, McEvoy P, Owaga ML, Odera JS, Ogutu B. Establishing a malaria diagnostics centre of excellence in Kisumu, Kenya. Malar J. 2007;6:79. doi: 10.1186/1475-2875-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Payne D. Use and limitations of light microscopy for diagnosing malaria at the primary health care level. Bull World Health Organ. 1988;66:621–626. [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins H, Asiimwe C, Bell D. Access to antimalarial therapy: accurate diagnosis is essential to achieving long term goals. BMJ. 2009;339:b2606. doi: 10.1136/bmj.b2606. [DOI] [PubMed] [Google Scholar]
- 16.Barnish G, Bates I, Iboro J. Newer drug combinations for malaria. BMJ. 2004;328:1511–1512. doi: 10.1136/bmj.328.7455.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nosten F, Ashley E. The detection and treatment of Plasmodium falciparum malaria: time for change. J Postgrad Med. 2004;50:35–39. [PubMed] [Google Scholar]
- 18.Barat L, Chipipa J, Kolczak M, Sukwa T. Does the availability of blood slide microscopy for malaria at health centers improve the management of persons with fever in Zambia? Am J Trop Med Hyg. 1999;60:1024–1030. doi: 10.4269/ajtmh.1999.60.1024. [DOI] [PubMed] [Google Scholar]
- 19.Reyburn H, Ruanda J, Mwerinde O, Drakeley C. The contribution of microscopy to targeting antimalarial treatment in a low transmission area of Tanzania. Malar J. 2006;5:4. doi: 10.1186/1475-2875-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]