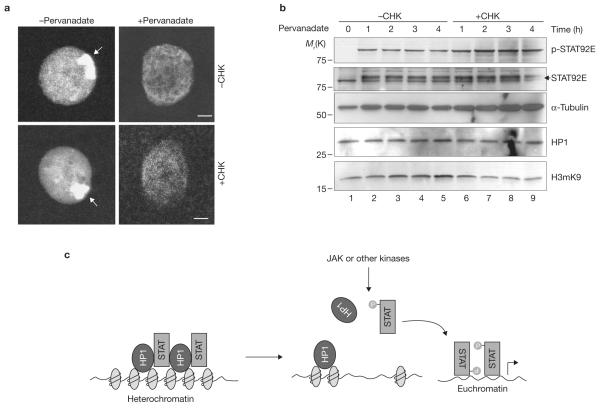
Figure 5.
Protein-synthesis-independent heterochromatin disruption following STAT92E phosphorylation. (a) Wild-type salivary glands were cultured with or without cycloheximide (CHX) for 1 h before addition of pervanadate for 1 h in vitro. Represented nuclei are shown. Note the disappearance of the HP1 foci in treated cells. (b) S2 cells were cultured with or without CHX for 1 h, and then stimulated with pervanadate for the indicated times. Cell extracts were blotted with the indicated antibodies. Note a greater increase in pSTAT92E (phosphorylated) and decrease in STAT92E (unphosphorylated) bands in CHX-treated samples, which is correlated with a greater decrease in H3mK9 levels. (c) A model for the role of STAT in heterochromatin stability. Unphosphorylated STAT is localized on heterochromatin in association with HP1. Increasing STAT phosphorylation (by JAK or other tyrosine kinases) reduces the amount of unphosphorylated STAT localized in heterochromatin. This in turn causes HP1 displacement from heterochromatin and heterochromatin instability. Dispersed phosphorylated STAT binds to cognitive sites in euchromatin to induce target-gene expression. Scale bars, 2 μm.