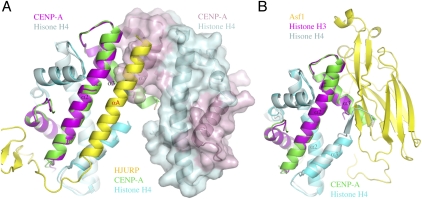
Figure 3.
HJURP binding prevents the formation of a CENP-A–histone H4 tetramer. (A) The structure of the HJURP–CENP-A–H4 complex is superimposed with the structure of the CENP-A–H4 tetramer (PDB ID: 3NQJ). (Left) The heterodimer from the tetramer structure superimposed with the HJURP complex is shown in a ribbon representation (CENP-A, magenta; H4, pale cyan), and the ribbon representation of the other heterodimer (CENP-A, light pink; H4, pale cyan) is overlaid with a surface representation. The HJURP complex is colored the same as in Figure 1A. (B) Histone chaperone Asf1 employs a different mechanism to block histone H3–H4 tetramer formation. The structure of the Asf1 complex (PDB ID: 2HUE) is shown in a ribbon diagram. (Yellow) Asf1; (magenta) H3; (pale cyan) H4. The H3–H4 dimer is aligned with the CENP-A–H4 heterodimer from the HJURP complex, the latter is positioned the same as in A but with HJURP removed.