Abstract
The mechanisms that regulate neuronal function are a sum of genetically determined programs and experience. The effect of experience on neuronal function is particularly important during development, because early-life positive and adverse experience (stress) may influence the still “plastic” nervous system long-term. Specifically, for hippocampal-mediated learning and memory processes, acute stress may enhance synaptic efficacy and overall learning ability, and conversely, chronic or severe stress has been shown to be detrimental. The mechanisms that enable stress to act as this “double-edged sword” are unclear. Here, we discuss the molecular mediators of the stress response in the hippocampus with an emphasis on novel findings regarding the role of the neuropeptide known as corticotropin-releasing hormone (CRH). We highlight the physiological and pathological roles of this peptide in the developing hippocampus, and their relevance to the long-term effects of early-life experience on cognitive function during adulthood.
Keywords: Corticotropin-releasing hormone, CRH, CRF, stress, development, hippocampus, rat, learning and memory, excitotoxicity, neuroplasticity
Input from the Environment Influences Neuronal and Circuit Function
In addition to intrinsic, genetically determined programs, inputs from the environment, or experience, regulate neuronal function. Thus, brain development and organization and the evolution of systems and circuits are governed by inherent, gene-dependent programs. Much has been unraveled about the genes that control neuronal fate, and differentiation and migration, and about the establishment and pruning of synaptic connectivity (1–3). These genetically determined programs provide a blueprint for the connectivity among neurons, the specificity of their input and responses, and the numerous other unresolved parameters that determine the function of the central nervous system (CNS) as a whole. Superimposed on this intricate, intrinsic blueprint, mechanisms that permit modulation by “experience” exist in the mammalian CNS. In other words, the CNS is designed with the capacity to be influenced, both immediately and long-term, by sensory input from within as well as the outside environment. This modulation, termed “plasticity,” involves altered expression and function of specific neuronal genes in discrete regions, cells, and synapses in response to a wide range of signals. For example, short-term neuronal plasticity (e.g., synaptic strengthening) is a key mechanism in long-term potentiation (LTP) and thus memory formation. Environmental input, including stress (4) and fear (5) can influence and enhance this process, leading to improved memory and increased chances for survival. Thus, stimulus-induced neuroplasticity, including the type that occurs upon stressful signals, may be advantageous. However, excessive or chronic adverse inputs have been shown to lead to long-term changes in neuronal function that can result in impaired synaptic activity (and in memory dysfunction). Our understanding of the mechanisms by which such stimuli (e.g., fear or stress) reach neurons and influence their function is incomplete, particularly in view of the remarkable progress that has been made in identifying the genes and proteins involved in regulating the genetic programs that govern brain development and maturation.
Environment-Driven Neuroplasticity is Most Critical During Development
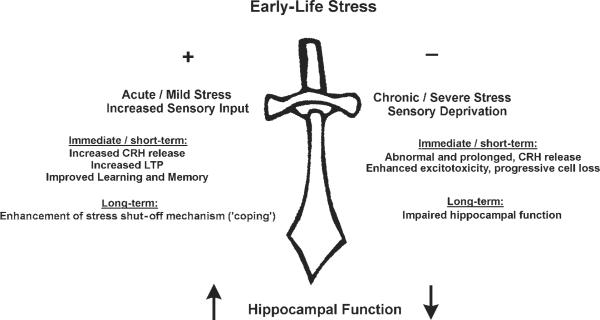
The effects of experience on neuronal activity are particularly important during development, when external stimuli may influence CNS function and interact with ongoing developmental programs to alter final neuronal “hard-wiring.” Indeed, during the first postnatal weeks, neuronal birth, differentiation, and migration are still ongoing (6–8), and neuronal connectivity—for example, in the hippocampus—is incomplete (9–12). Do early life experiences, both “positive” (e.g., environmental enrichment) or “negative” (severe stress), alter the structure and the function of the hippocampus? And if so, what are the mechanisms? In this article, we discuss these questions, and provide evidence that “positive” and “negative” experience utilize common molecular mechanisms, activated to differing degrees, to influence the hippocampus both acutely and in the long term (Fig. 1).
Fig. 1.
Hippocampal function, both short-term (e.g., LTP), and long-lasting (e.g., neuronal survival) is influenced by early-life experience. The figure highlights the role of the neuromodulator corticotropin-releasing hormone (CRH) in the mechanisms that mediate this “double-edged sword” effect.
Evidence for Long-Term Effects of Early-Life Experience on Neuronal Function in the Hippocampus: The Double-Edged Sword
Extensive evidence from human and animal studies documents the profound effects of early-life experience on hippocampus-mediated memory functions during adulthood. These studies demonstrate several principles: Importantly, the nature and magnitude of the inciting environmental stimuli determine whether their long-term effects will enhance or impair hippocampal function (Fig. 1). For example, on the “positive edge of the sword,” maternal contact during development promotes cognitive function later in life in rats as well as primates (13,14). Environmental enrichment also improves hippocampal function, perhaps by inducing neurogenesis and increasing the survival of newly formed granule cells (15). Furthermore, environmental enrichment enhances Morris water maze performance, a measure of hippocampus-dependent learning and memory function, in both adult and developing rats. Remarkably, the magnitude of the effect is greatest during the developmental period, suggesting a greater extent of neuro-plasticity during this age (16).
Along the “sinister” edge of the sword, a large body of evidence supports the notion that adverse experience (e.g., severe stress) early in life results in impaired hippocampal function and structure. In humans, it has been well-documented that neglect and abuse during childhood correlate with a higher incidence of learning disabilities later in life, implying hippocampus-mediated learning and memory deficits (17). Studies using magnetic resonance imaging (MRI) indicate that adults who are subjected to abuse early in life have smaller average hippocampal volumes compared with age-matched controls (18,19). Animal studies in nonhuman primates and rats are in general agreement with this human data. In primates, rearing monkeys in isolation (with no maternal or sibling contact) results in learning and memory deficits but no hippocampal volume loss (14,20). In addition, reproducing the neurohormonal profiles that occur during stress via administration of stress-induced hormones to developing primates or rats can mimic the effects of adverse early-life experience, including delayed neurodevelopment (21), memory deficits, and loss of hippocampal pyramidal cells (22,23). The ability of stress hormones to reproduce the effects of early-life stress supports their mechanistic role in stress-induced hippocampal dysfunction. Indeed, a common denominator of early-life stimuli that modulate hippocampal systems positively or adversely is their activation of neuroendocrine and neuronal stress-induced molecular cascades (20,24).
Putative Mechanisms for Experience-Dependent Modulation of Hippocampal Function
Stress is defined operationally as signals from within or outside the organism that activate the stress-response machinery. What are the molecules and circuits that are activated by stressful signals? In rodents and primates, stress activates two major pathways, including the hypothalamic pituitary adrenal (HPA) axis and the limbic-neuroendocrine circuit. Activation of the HPA axis in response to stressful stimuli involves rapid secretion of corticotropin-releasing hormone (CRH) from terminals of peptidergic neurons in the paraventricular nucleus of the hypothalamus (PVN) to influence the release of adrenocorticotrophic hormone (ACTH) from the corticotrophs of the anterior pituitary. Once secreted, ACTH travels through the bloodstream and induces the adrenal glands to release glucocorticoids. Systemically, glucocorticoids increase available energy that is necessary for rapid adaptation to acute stress; centrally, they provide regulatory feedback to the HPA axis via activation of glucocorticoid receptors in the PVN, amygdala, and hippocampus (25–30).
Interestingly, this stress-response, including rapid release and especially the increased expression of hypothalamic CRH, also occurs during experiences that would not a priori be considered “stressful.” For example, removal of a neonatal rat from its cage (such as occurs during the handling paradigm) suffices to induce expression of the CRH gene (31,32). Thus, if “stress” is defined as an experience that activates the CRH-ACTH glucocorticoid cascade, then early-life experiences including handling and other changes in the environment, might be considered mild stressors (Fig. 1).
The second major stress-mediating circuit consists of limbic pathways that are more sensitive to stressors involving higher-order sensory processing (29). Based on immediate early gene analyses and lesion studies, these limbic pathways primarily involve propagation and integration of stress responses in the amygdala and hippocampus (33–36). Specifically, the central nucleus of the amygdala (ACe) is a key region involved in regulating the central stress response: stimulating this nucleus reproduces stress behaviors (37), whereas ablating it eliminates stress-induced release of CRH from PVN (38). There is abundant evidence that subsequent to integration in the amygdala, information regarding “emotional” or “cognitive” stress, but not some types of physical stress (39), reaches the hippocampus. First, enhanced memory consolidation observed in aversive learning paradigms (which activate the HPA axis) requires activation of glucocorticoid receptors in both the amygdala and hippocampus (4). Second, lesions of the basolateral nucleus of the amygdala (BLA) block the memory-modulating effects of glucocorticoids on the hippocampus, suggesting that amygdala precedes hippocampus in the circuit involved in stress-related information (40). Once they reach the hippocampus, stress-related signals enhance hippocampal LTP (41,42), and activate the immediate early gene c-fos in hippocampal neurons in a stressor-specific manner (36). These observations indicate that glucocorticoids contribute to activation of stress-induced changes in the amygdala and hippocampal neuronal function. However, more recent information suggests that in addition to these well-established stress hormones, the neuropeptide CRH is a likely contributor to the activation—and modulation—of these structures in response to stress.
CRH-expressing neurons and CRH receptors are found in amygdala nuclei, which are key components of the limbic stress circuit (43–46). ACe contains a high concentration of CRH (44), and CRH receptors are concentrated in the basolateral nucleus of the amygdala (BLA) (46,47). Stress triggers the release of endogenous CRH in ACe (48,49,50a), and administration of CRH antagonists into ACe can attenuate stress-induced behaviors (50). Relay stations that connect amygdala outflow nuclei (e.g., lateral and basal) to the hippocampus also contain CRH-expressing neurons. These include the entorhinal cortex (51) and bed nucleus of the stria terminalis (BNST) (44). In the hippocampal formation itself, early work described the presence of small numbers of CRH-containing interneurons (52,53). It should be noted that these studies focused on the mature hippocampus. In the developing hippocampus, recent studies from our laboratory have identified and characterized a large population of CRH-expressing interneurons that directly innervate cell bodies of hippocampal pyramidal cells, thus significantly influencing their activity (54,55; Fig. 2). Indeed, the developmental profile of this robust CRH-expressing cell population is consistent with a key role for the peptide in mediating the beneficial and adverse effects of early-life stress on hippocampal neurons (42,55).
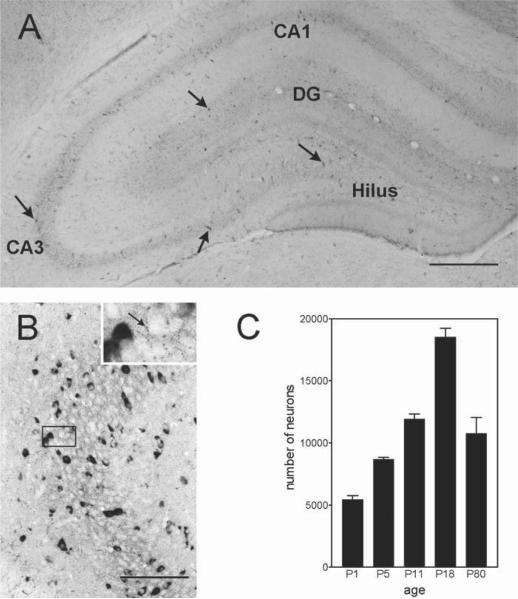
Fig. 2.
Expression pattern of CRH-secreting neurons in the hippocampal formation. (A) CRH-expressing neurons (arrows) are particularly common in the principal cell layers of the hippocampus, as shown in the immunocytochemistry photomicrograph from an 18-d-old rat. (B) CRH-expressing cells are interneurons as evident from the robust expression of GAD (glutamic acid decarboxylase, the GABA-synthesizing enzyme), seen as dark signal in the in situ hybridization reaction. These CRH-expressing interneurons form “baskets” of processes around the cell bodies of the pyramidal cells (inset), innervating them directly. The age-dependent profile of CRH-expressing cells in the hippocampus is shown in panel (C). Total numbers of CRH neurons increase progressively from P1 to P18, with a subsequent decline to adult levels. Scale bar = 700 μm for A, 150 μm for B, and 50 μm for inset.
Is CRH Involved in the Mechanisms by Which Early-Life Experience Improves Hippocampal Function Long-Term?
Early Life Experience Improves Hippocampal Function Long-Term
Evidence from human and animal studies suggests that maternal contact and interaction with peers and siblings are vital for normal functional development of the hippocampus (14,20,56,57). Deprivation of either of these factors may lead to physiological alterations, resulting in impairment of hippocampus-dependent learning and memory. As expected, experimental paradigms designed to enhance these positive environmental factors during the critical developmental period (roughly postnatal days 2–10 in the rat) permanently modify the HPA axis (58,59), leading to better “coping” with stressful stimuli during adulthood. For example, daily handling of neonatal rats leads to a significant attenuation and shortening of the stress response later in life, compared with animals raised with no disturbance (59–62). In addition to better stress-coping mechanisms, adult, neonatally handled rats exhibit improved spatial memory acquisition skills when evaluated in the Morris water maze (24). The positive effects of early-life handling have been found to depend on increased maternal licking and grooming of the handled pups upon their return to their home cages (13,63). However, the mechanisms by which increased maternal sensory input influences specific molecules and circuits in the pups' brains and the nature of these key molecules have remained a mystery (23,59,64). Intuitively, transmitters and hormones that are induced during stress and contribute to the limbic stress response (57,65) constitute excellent candidates to transduce the “stress experience” into short- or long-term molecular and functional changes of this system (66). Therefore, we consider the role of alterations in hippocampal and potentially hypothalamic glucocorticoid receptors (GR) as well as levels and/or function of CRH and CRH receptors (23,24,58) in these results of early-life experience.
In fact, significant changes in both hippocampal and cortical GR, and in hypothalamic CRH, are found in adult rats that have been handled early in life. Hippocampal GR levels are increased, permitting a more efficient “shut-off” mechanism for the stress response (62). Thus, the elevated GR mRNA levels and GR binding are consistent with the enhanced negative feedback (e.g., “shut-off”), and shortened stress response in these adult rats (62). A role for CRH has also been demonstrated: Adults who experience neonatal handling express less CRH in the PVN (58,59). This would predict attenuated CRH release (measured by ACTH and corticosterone plasma levels), as was demonstrated in these animals (58,59). In summary, changes in both hippocampal GR and in hypothalamic CRH may contribute to the attenuated response to stress in adults who were handled as neonates (58,59,67). However, the precise chain of events, and the specific mechanisms by which the early-life-handling process provokes these profound life-long changes has remained elusive. Understanding the sequence of changes leading from early-life experience to overall better “coping” with stress throughout life is of tremendous clinical importance, because it can suggest therapeutic or interventional strategies for stress-related disorders (24).
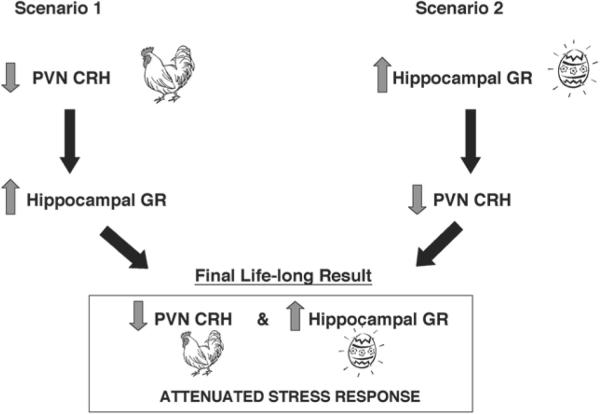
How does neonatal handling (or the resulting increased maternal sensory input) lead to the persistent increase of hippocampal GR and reduction of hypothalamic CRH? Although it has often been suggested that the primary, early effect of maternal sensory input is at the level of the hippocampus, directly increasing GR expression (68), most of the current evidence suggests an alternative scenario. Recent studies have demonstrated that daily neonatal handling suppresses hypothalamic CRH mRNA expression prior to the increase in hippocampal GR expression (59) (Fig. 3). This suggests that alteration in CRH levels, induced by the handling-provoked enhanced maternal sensory input, may be a key early event. Thus, reduced CRH expression could be an early, primary change that leads to a cascade of downstream molecular alterations—including increased GR expression in the hippocampus—which ultimately affect hippocampal function. The proposed sequence of this cascade suggests that lower levels of hypothalamic CRH lead to diminished CRH release during stress, with subsequent reduction of glucocorticoid secretion. Lower plasma (and thus brain) glucocorticoid levels disinhibit (upregulate) hippocampal GR expression (69). This cascade leads to a new molecular and functional steady-state, constituting the reduced HPA axis tone observed in adult rats handled during early life. Importantly, the increased expression of GR in the hippocampal formation may also alter synaptic activity and memory function (70–72).
Fig. 3.
Alternative mechanisms by which early-life-handling in rats leads to an attenuated stress response in adulthood. The authors emphasize that the precedence of the “chicken” (reduced CRH expression in hypothalamic paraventricular nucleus [PVN]) over the “egg” (increased expression of glucocorticoid receptors [GR] in hippocampus) is specific to the process under discussion.
Direct Effects of CRH on Hippocampal Neurons
In the scenario depicted in the previous section, early-life experience influences hippocampal function indirectly, via initial alterations of CRH expression in the hypothalamus. However, is hypothalamic CRH expression alone regulated by early-life experience? Could neonatal stress or experience also elicit rapid changes of CRH expression in the hippocampus?
Evidence for selective modulation of CRH mRNA expression in hippocampus by specific early-life stimuli has recently been provided (36). In the hippocampal formation, CRH is primarily expressed in interneurons, which are interspersed throughout the pyramidal-cell layer, where they innervate the principal cells (54,55). Thus, alteration of CRH synthesis and subsequent release from these interneurons can directly influence the function of hippocampal pyramidal neurons (42,73). Within the hippocampus, the postsynaptic actions of CRH are excitatory (31). Intracellular electrophysiological recordings from hippocampal CA1 and CA3 pyramidal neurons of the adult rat revealed that exogenous, applied CRH increased the firing of CA1 pyramidal neurons in response to excitatory input, and reduced afterhyperpolarization following an action-potential train elicited by depolarizing current (74). In immature slices, CRH led to hyperexcitability of CA3 pyramidal neurons, resulting in a net increase of glutamate release and enhancement of synaptic efficacy (73). Indeed, the peptide enhances LTP, thus facilitating memory retention (42,75–77). Importantly, recent studies have documented the effects of endogenous CRH, which was released during stress, on hippocampal synaptic plasticity (42). Thus, selective stresses elicit release of endogenous hippocampal CRH, and the endogenous peptide facilitates hippocampal function (42).
CRH exerts its excitatory influence on hippocampal pyramidal cells by activating post-synaptic G-protein-coupled membrane-bound receptors. Two receptor subtypes for this peptide (CRF1 and CRF2) have been identified and characterized, and their differential anatomical distribution suggests distinct functional roles (45–47,78). Using selective non-peptide antagonists, our laboratory found that CRF1 is the receptor mediating the excitatory effects of CRH in hippocampus (28), which is strongly expressed in the hippocampal pyramidal-cell layer (46). Activation of the CRF1 receptor induces the cAMP cascade, leading to phosphorylation of the cAMP-response element-binding protein (CREB). The phosphorylated form of CREB (pCREB) regulates the transcription of genes containing the cAMP-response element (CRE), such as the immediately early gene c-fos as well as CRH itself (79). CRH synthesis and release influences hippocampal function as discussed here. In addition, C-fos expression in CRF1-expressing neurons (80) should lead to activation of many diverse molecular cascades, which may further influence neuronal function. Importantly, we found that blockage of CRH receptors, which prevents stress signals from inducing c-fos expression in CRF1-bearing hippocampal neurons (80a).
The Effects of CRH on Hippocampal Neurons are Most Potent Early in Life
Hippocampal-slice studies have demonstrated enhanced excitatory properties of CRH in the immature compared with the adult hippocampus (73,81). These observations are further strengthened by in vivo studies showing that picomolar amounts of CRH administered into the lateral ventricle of infant rats produce severe limbic seizures that last for several hours (82), and a much higher dose (200-fold or 50-fold if calculated per g of brain weight) is required to elicit excitatory discharges in the adult (83,84). In addition, administration of CRH to immature rats has been shown to injure hippocampal neurons in a pattern that is highly reminiscent of that found in stress-induced injury (23,85), yet administration of equivalent doses to the adult does not produce neuronal injury. This finding is particularly striking because other excitatory compounds, such as kainic acid, which injures neurons in the adult, cause similarly severe seizures in immature animals, but fail to provoke excitatory neuronal death (86,87). A partial explanation for this age-dependent powerful effect of CRH on hippocampal neurons may involve the abundance of target receptors—studies using in situ hybridization as well as receptor binding have shown that CRF1-receptor levels peak in the hippocampus during development (47,88). Importantly, CRH may enhance neuronal excitability for a prolonged period in the immature rat. Repeated administration of CRH resulted in striking augmentation of the excitatory potency of the subsequently administered kainic acid (89). Thus, one can propose a scenario in which stress leads to enhanced hippocampal CRH levels, which “primes” both the excitatory and excitotoxic influences of glutamate (31,73). Indeed, CRH-receptor antagonists have been shown to ameliorate glutamate-induced neurotoxicity, suggesting a role for endogenous CRH in this excitotoxic mechanism (90–92; see also 93). The findings cited here demonstrate that CRH in pathological amounts (although still in the picomolar range) can contribute to neuronal injury in the immature hippocampus. Are hippocampal pyramidal cells exposed to such “excessive” levels of CRH during stress? Does peptide-mediated excitotoxicity contribute to the adverse effects of early-life stress on hippocampal neuronal function and integrity?
Is CRH Involved in the Mechanisms by Which Severe, Chronic Early-Life Stress Impairs Hippocampal Function Long-Term
As mentioned previously, severe or chronic stress during development can produce adverse effects on the hippocampus, impairing hippocampal function permanently. Candidate mechanisms for such long-term effects include signaling processes that have been found to be induced by stressful challenges in the immature CNS. The major stress hormones mediating these molecular cascades are CRH and glucocorticoids, and it has been shown previously that saturation of GRs by “stress levels” of glucocorticoids results in hippocampal neuronal injury (94). However, GRs reside primarily in CA1, (95) whereas stress-induced damage involves mainly CA3 pyramidal cells (96). In addition, glucocorticoids fail to reproduce the effects of stress on hippocampal integrity when administered in a manner that is not stressful to the animal (e.g., in food), suggesting that other factors may be involved (97).
We reasoned that if CRH is one of the stress-activated factors that contributes to the mechanism by which early-life stress causes long-lasting impairments of hippocampal function and integrity, then early-life administration of the peptide should reproduce these deficits. Indeed, a recent study from our laboratory supports this prediction. Significant structural and functional hippocampal deficits were found in adult rats given CRH early in life (postnatal d 10) (23). Structurally, the adult rats treated with CRH during development had a significantly lower number of CA3 hippocampal pyramidal cells as compared to vehicle-treated age-matched controls. This loss of pyramidal cells appeared to be progressive, because at 12 mo, CRH-administered animals diverged more from age-matched controls compared with the 8-mo time-point. The loss of CA3 pyramidal cells was also reflected by altered growth patterns of the mossy fibers, the axons of the dentate gyrus granule cells that normally innervate these CA3 neurons. This exuberant growth (“sprouting”) is consistent with, and typical of, a loss of normal targets of the mossy fibers (98). Importantly, the synapses formed by the aberrant mossy fibers on the remaining CA3 pyramidal cells are excitatory (glutamatergic), which could promote further excitotoxic injury to these neurons.
In addition to these structural changes, administration of CRH to developing rats induced hippocampus-mediated learning and memory impairments that were similar to those induced by early-life stress (14,23). Specifically, deficits in spatial memory acquisition skills, measured using the Morris water maze test, were observed in adult rats given CRH early in life compared to controls. Short-term memory deficits were also detected in these animals using the nonaversive object-recognition test. Importantly, both of these aspects of memory function are hippocampus-dependent. As shown previously for the cell loss, the decline in hippocampal function in the CRH-treated animals was progressive, and worsened over time.
Does the Effect of Early-Life CRH Administration on the Hippocampus Require Glucocorticoids?
CRH induces the release of endogenous glucocorticoids, which in high (“stress”) levels may impact hippocampal neurons via activation of GRs (99). Therefore, the possibility that early-life administration of CRH exerted its long-term effects on hippocampal structure and function indirectly—e.g., via glucocorticoids—was investigated: CRH was given to immature rats rendered devoid of endogenous steroids (adrenalectomized). Glucocorticoid levels were then maintained at levels much lower than those seen during stress (“clamped”) by supplementing the drinking water. In these animals, in which saturation of hippocampal GR did not occur, early-life administration of CRH still caused significant loss of hippocampal pyramidal cells and impairment of hippocampus-mediated learning. These findings indicate that high plasma glucocorticoid levels are not required for the anatomical and cognitive adverse effects of early-life CRH administration.
Why would a single administration of CRH to the infant rat produce a progressive loss of hippocampal neurons and hippocampus-dependent cognitive function? Further insight into the mechanisms involved was provided through the analysis of CRH and CRF1 expression in the hippocampus after early-life administration of the peptide. Adult rats given CRH early in life had significantly elevated CRH and CRF1 mRNA steady-state levels compared to controls. Thus, CRH administration to immature rats approximated the pattern of hippocampal-cell activation provoked by stress (33,100), and led to chronic elevation of CRH synthesis in hippocampal interneurons, as shown for certain early-life stressors (36). Enhanced CRH levels in the hippocampus resulted in increased stress-induced release of the peptide from terminals that innervate CA3 pyramidal cells. This, coupled with upregulated levels of the CRF1 receptor, promoted the overall synaptic effects of CRH. High levels of synaptic CRH, combined with the increased glutamate release from the observed excitatory aberrant mossy-fiber synapses (Fig. 4), probably contributed to excitotoxic injury (31,85,90,92). Surviving neurons formed targets for further mossy fiber sprouting and the formation of new excitatory synapses, perpetuating this vicious excitotoxic cycle (23). Not surprisingly, progressive hippocampal-cell loss was reflected in progressive deficits in hippocampus-mediated learning and memory tasks (23).
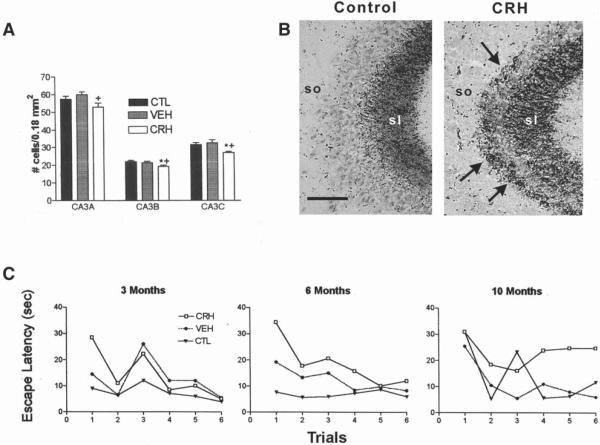
Fig. 4.
CRH administration to neonatal rats reproduces the structural and functional hippocampal deficits induced by early-life stress. (A) Cell numbers in subregions of the CA3 pyramidal-cell layer from CRH-treated, vehicle-treated, and naive controls were determined at age 12 mo. One-way ANOVA with Tukey's multiple analysis indicated significant (p < 0.05; *vs naive; + vs vehicle-treated) neuronal loss in CRH-treated rats in all CA3 subregions at 12 mo of age. (B) Sections of CA3A pyramidal cell regions from vehicle- and CRH- treated rats (sacrificed at 12 mo), subjected to Timm's stain for visualizing the high zinc content of mossy fiber (axons of the CA3-innervating granule cells) terminals. In CRH-treated rats, these terminals were abnormally abundant within CA3 stratum oriens (so). (C) CRH-treated rats show a trend toward impaired performance (increased escape latency) using the Morris water maze at age 3 mo. By 6 and 10 mo, rats treated with CRH early in life take significantly longer to locate the hidden platform (two-way ANOVA, treatment effect at 6 mo: F2,132 = 9.62, p < 0.001; at 10 mo; F2,132 = 5.53, p < 0.01). sl, stratum lucidum. Scale bar = 50 μm. These data are modified and reproduced from (ref. 23).
Summary
Early-life experience, particularly stress, influences neuronal function in the hippocampus both acutely and long-term. Here, we discuss recent data supporting the notion that CRH may be a critical contributor to these processes. CRH is a neuropeptide, and members of this family of neurohormones exert their effects over a greater distance and longer time period compared to traditional neurotransmitters. The effects of neuropeptides generally last for a period of minutes to hours rather than seconds, probably because mechanisms for their clearance from the synaptic cleft (e.g., transporters or rapid degradation) are less prevalent. This time frame is optimal for mechanisms of memory acquisition, such as LTP. In contrast to the beneficial effects of physiological CRH release, the prolonged actions of abnormally high levels of CRH, released under pathological conditions, may promote excitotoxicity during an age when activity-induced excitotoxicity is rare (31,101). The nature of these detrimental effects of the peptide, and their critical role in mediating the clinically important adverse effects of early-life stress on hippocampal-cell viability and function, have been highlighted in this review.
References
- 1.Aamodt SM, Constantine-Paton M. The role of neural activity in synaptic development and its implications for adult brain function. Adv. Neurol. 1999;79:133–144. [PubMed] [Google Scholar]
- 2.Rubenstein JLR, Rakic P. Genetic control of cortical development. Cereb. Cortex. 1999;9:521–523. doi: 10.1093/cercor/9.6.521. [DOI] [PubMed] [Google Scholar]
- 3.Monuki ES, Walsh CA. Mechanisms of cerebral cortical patterning in mice and humans. Nat. Neurosci. Suppl. 2001;4:1199–1206. doi: 10.1038/nn752. [DOI] [PubMed] [Google Scholar]
- 4.Roozendaal B, Quirarte GL, McGaugh JL. Stress-activated hormonal systems and the regulation of memory storage. Ann. NY Acad. Sci. 1997;821:247–258. doi: 10.1111/j.1749-6632.1997.tb48284.x. [DOI] [PubMed] [Google Scholar]
- 5.Schafe GE, Nader K, Blair HT. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- 6.Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J. Comp. Neurol. 1990;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- 7.Cameron HA, Gould E. The control of neuronal birth and survival. In: Shaw CA, editor. Receptor Dynamics in Neural Development. CRC Press; New York, NY: 1996. pp. 141–157. [Google Scholar]
- 8.Bender RA, Lauterborn JC, Gall CM, Cariaga W, Baram TZ. Enhanced CREB phosphorylation in immature dentate gyrus granule cells precedes neurotrophin expression and indicates a specific role of CREB in granule cell differentiation. Eur. J. Neurosci. 2001;13:679–686. doi: 10.1046/j.1460-9568.2001.01432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amaral DG, Dent JA. Development of mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansion. J. Comp. Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- 10.Ribak CE, Navetta MS. An immature mossy fiber innervation of hilar neurons may explain their resistance to kainate-induced cell death in 15-day-old rats. Dev. Brain Res. 1994;79:47–62. doi: 10.1016/0165-3806(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 11.Tamamaki N. Development of afferent fiber lamination in the infrapyramidal blade of the rat dentate gyrus. J. Comp. Neurol. 1999;411:257–266. [PubMed] [Google Scholar]
- 12.Swann JW, Smith KL, Lee CL. Neuronal activity and the establishment of normal and epileptic circuits during brain development. Int. Rev. Neurobiol. 2001;45:89–118. doi: 10.1016/s0074-7742(01)45007-0. [DOI] [PubMed] [Google Scholar]
- 13.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- 15.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 16.Williams BM, Luo Y, Ward C, Redd K, Gibson R, Kuczaj SA, et al. Environmental enrichment: effects on spatial memory and hippocampal CREB immunoreactivity. Physiol. Behav. 2001;73:649–658. doi: 10.1016/s0031-9384(01)00543-1. [DOI] [PubMed] [Google Scholar]
- 17.Trickett PK, McBride-Chang C. The developmental impact of different forms of child abuse and neglect. Dev. Rev. 1995;15:311–337. [Google Scholar]
- 18.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol. Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol. Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev. and Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 21.Flagel SB, Vazquez DM, Watson SJ, Jr., Neal CR., Jr. Effects of tapering neonatal dexamethasone on rat growth, neurodevelopment, and stress response. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:55–63. doi: 10.1152/ajpregu.2002.282.1.R55. [DOI] [PubMed] [Google Scholar]
- 22.Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, et al. Neurotoxicity of glucocorticoids in the primate brain. Horm. Behav. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- 23.Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc. Natl. Acad. Sci. USA. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meaney MJ, Aitken DH, Van Berkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- 25.Keller-Wood M, Dallman M. Corticosteroid inhibition of ACTH secretion. Endocr. Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- 26.Sawchenko PE. Evidence for a local site of action for glucocorticoids in inhibiting CRF and vasopressin expression in parvocellular neurosecretory neurons. Brain Res. 1987;403:213–224. doi: 10.1016/0006-8993(87)90058-8. [DOI] [PubMed] [Google Scholar]
- 27.de Kloet ER, De Kock S, Schild V, Veldhuis HD. Antiglucocorticoid RU 38486 attenuates retention of a behavior and disinhibits the hypothalamic-pituitary-adrenal axis at different sites. Neuroendocrinol. 1988;47:109–115. doi: 10.1159/000124900. [DOI] [PubMed] [Google Scholar]
- 28.Baram TZ, Chalmers DT, Chen C, Koutsoukos Y, De Souza EB. The CRF1 receptor mediates the excitatory actions of corticotropin releasing factor (CRF) in the developing rat brain: in vivo evidence using a novel, selective, non-peptide CRF receptor antagonist. Brain Res. 1997;770:89–95. doi: 10.1016/s0006-8993(97)00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herman JP, Cullinan WE. Neuro-circuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 30.Lopez JF, Akil H, Watson SJ. Neural circuits mediating stress. Biol. Psychiatry. 1999;46:1461–1471. doi: 10.1016/s0006-3223(99)00266-8. [DOI] [PubMed] [Google Scholar]
- 31.Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Hatalski CG, Brunson KL, Baram TZ. Rapid phosphorylation of the CRE binding protein precedes stress-induced activation of the corticotropin releasing hormone gene in medial parvocellular hypothalamic neurons of the immature rat. Mol. Brain Res. 2002;96:39–49. doi: 10.1016/s0169-328x(01)00265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imaki T, Shibasaki T, Hotta M, Demura H. Intracerebroventricular administration of corticotropin-releasing factor induces c-fos mRNA expression in brain regions related to stress responses: comparison with pattern of c-fos mRNA induction after stress. Brain Res. 1993;616:114–125. doi: 10.1016/0006-8993(93)90199-w. [DOI] [PubMed] [Google Scholar]
- 34.McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: interaction with other brain systems. Proc. Natl. Acad. Sci. USA. 1996;93:13,508–13,514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatalski CG, Guirguis C, Baram TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated stress in the immature rat. J. Neuroendocrinol. 1998;10:663–669. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatalski CG, Brunson KL, Tantayanubutr B, Chen Y, Baram TZ. Neuronal activity and stress differentially regulate hippocampal and hypothalamic corticotropin releasing hormone expression in the immature rat. Neuroscience. 2000;101:571–580. doi: 10.1016/s0306-4522(00)00386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tannahill LA, Sheward WJ, Robinson IC, Fink G. Corticotropin-releasing factor-41, vasopressin and oxytocin release into hypophysial portal blood in the rat: effects of electrical stimulation of the hypothalamus, amygdala and hippocampus. J. Endocrinol. 1991;129:99–107. doi: 10.1677/joe.0.1290099. [DOI] [PubMed] [Google Scholar]
- 38.Beaulieu S, Pelletier G, Vaudry H, Barden N. Influence of the central nucleus of the amygdala on the content of corticotropin-releasing factor in the median eminence. Neuroendocrinology. 1989;49:255–261. doi: 10.1159/000125125. [DOI] [PubMed] [Google Scholar]
- 39.Cullinan WE, Helmreich DL, Watson SJ. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J. Comp. Neurol. 1996;368:88–99. doi: 10.1002/(SICI)1096-9861(19960422)368:1<88::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 40.Roozendaal B, McGaugh JL. Baso-lateral amygdala lesions block the memory enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur. J. Neurosci. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 41.Garcia R, Tocco G, Baudry M, Thompson RF. Exposure to a conditioned aversive environment interferes with long-term potentiation induction in the fimbria-CA3 pathway. Neuroscience. 1998;82:139–145. doi: 10.1016/s0306-4522(97)00285-6. [DOI] [PubMed] [Google Scholar]
- 42.Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J. Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors are widely distributed within the rat CNS: an autoradiographic study. J. Neurosci. 1985;5:3189–3203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Crit. Rev. Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- 45.Eghbal-Ahmadi M, Hatalski CG, Lovenberg TW, Avishai-Eliner SA, Chalmers DT, Baram TZ. The developmental profile of the corticotropin releasing hormone receptor (CRF2) in rat brain predicts distinct agespecific functions. Dev. Brain Res. 1998;107:81–90. doi: 10.1016/s0165-3806(98)00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y, Brunson K, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 analysis using an antibody directed against the C-terminus. J. Comp. Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avishai-Eliner SA, Yi SJ, Baram TZ. Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the limbic system. Dev. Brain Res. 1996;91:159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalin NH, Takahashi LK, Chen F-L. Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 1994;656:182–186. doi: 10.1016/0006-8993(94)91382-x. [DOI] [PubMed] [Google Scholar]
- 49.Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J. Neurosci. 1998;18:4758–4766. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swiergel AH, Takahashi LK, Kalin NH. Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res. 1993;623:229–234. doi: 10.1016/0006-8993(93)91432-r. [DOI] [PubMed] [Google Scholar]
- 50a.Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc. Nat. Acad. Sci. 2002;99:13,908–13,913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piekut DT, Phipps B. Increased corticotropin-releasing factor immunoreactivity in select brain sites following kainate elicited seizures. Brain Res. 1998;781:99–111. doi: 10.1016/s0006-8993(97)01219-5. [DOI] [PubMed] [Google Scholar]
- 52.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 53.Sakanaka M, Shibasaki T, Lederis K. Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzadine method. J. Comp. Neurol. 1987;260:256–298. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- 54.Yan XX, Toth Z, Schultz L, Ribak CE, Baram TZ. Corticotropin releasing hormone (CRH)-containing neurons in the hippocampal formation: morphological and neurochemical characterization. Hippocampus. 1998;8:231–243. doi: 10.1002/(SICI)1098-1063(1998)8:3<231::AID-HIPO6>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y, Bender RA, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J. Neurosci. 2001;21:7171–7181. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- 57.Vazquez DM, Lopez JF, Van Hoers H, Watson SJ, Levine S. Maternal deprivation regulates serotonin 1A and 2A receptors in the infant rat. Brain Res. 2000;855:76–82. doi: 10.1016/s0006-8993(99)02307-0. [DOI] [PubMed] [Google Scholar]
- 58.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol. Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 59.Avishai-Eliner S, Eghbal-Ahmadi M, Tabachnik E, Brunson KL, Baram TZ. Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology. 2001;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levine S, Lewis G. Critical period for the effects of infantile experience on the maturation of a stress response. Science. 1959;129:42–43. doi: 10.1126/science.129.3340.42. [DOI] [PubMed] [Google Scholar]
- 61.Hess JL, Denenberg VH, Zarrow M, Peiffer WD. Modification of the corticosterone response curve as a function of handling in infancy. Physiol. Behav. 1969;4:102–109. [Google Scholar]
- 62.Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev. Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 63.Francis DD, Meaney MJ. Maternal care and the development of stress responses. Curr. Opin. Neurobiol. 1999;9:128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- 64.Eghbal-Ahmadi M, Avisai-Eliner S, Hatalski CG, Baram TZ. Regulation of the expression of corticotropin releasing factor receptor type 2 (CRF2) in the hypothalamus and amygdala of the immature rat. J. Neurosci. 1999;19:3982–3991. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lopez JF, Liberzon I, Vazquez DM, Young EA, Watson SJ. Serotonin 1A receptor messenger RNA regulation in the hippocampus after acute stress. Biol. Psychiatry. 1999;45:934–937. doi: 10.1016/s0006-3223(98)00224-8. [DOI] [PubMed] [Google Scholar]
- 66.Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. USA. 2001;98:12,796–12,801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Viau V, Sharma S, Plotsky PM, Meaney MJ. The hypothalamic-pituitary-adrenal response to stress in handled and non-handled rats: differences in stress-induced plasma ACTH secretion are not dependent upon increased corticosterone levels. J. Neurosci. 1993;13:1097–1105. doi: 10.1523/JNEUROSCI.13-03-01097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meaney MJ, Aitken DH. The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: temporal parameters. Brain Res. 1985;354:301–304. doi: 10.1016/0165-3806(85)90183-x. [DOI] [PubMed] [Google Scholar]
- 69.Herman JP, Patel PD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralcorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol. Endocrinol. 1997;3:3072–3082. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- 70.Joels M, de Kloet ER. Control of neuronal excitability by corticosteroid hormones. Trends Neurosci. 1992;15:25–30. doi: 10.1016/0166-2236(92)90345-9. [DOI] [PubMed] [Google Scholar]
- 71.Pavlides C, Watanabe Y, Magarinos AM, McEwen BS. Opposing roles of type I and type II adrenal steroid receptors in hippocampal long-term potentiation. Neuro-science. 1995;68:387–394. doi: 10.1016/0306-4522(95)00151-8. [DOI] [PubMed] [Google Scholar]
- 72.Joels M. Corticosteroid actions in the hippocampus. J. Neuroendocrinol. 2001;13:657–669. doi: 10.1046/j.1365-2826.2001.00688.x. [DOI] [PubMed] [Google Scholar]
- 73.Hollrigel GS, Chen K, Baram TZ, Soltesz I. The pro-convulsant actions of corticotropin-releasing hormone in the hippocampus of infant rats. Neuroscience. 1998;84:71–79. doi: 10.1016/s0306-4522(97)00499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–877. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- 75.Lee EH, Hung HC, Lu KT, Chen WH, Chen HY. Protein synthesis in the hippocampus associated with memory facilitation by corticotropin-releasing factor in rats. Peptides. 1992;13:927–937. doi: 10.1016/0196-9781(92)90051-4. [DOI] [PubMed] [Google Scholar]
- 76.Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, et al. Displacement of CRF from its binding protein as a possible treatment for Alzheimer's disease. Nature. 1995;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- 77.Lee EH, Huang AM, Tsuei KS, Lee WY. Enhanced hippocampal corticotropin-releasing factor gene expression associated with memory consolidation and memory storage in rats. Chin. J. Physiol. 1996;39:197–203. [PubMed] [Google Scholar]
- 78.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J. Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hatalski CG, Baram TZ. Stress-induced transcriptional regulation in the developing rat brain involves increased cyclic adenosine 3′, 5′-monophosphate-regulatory element binding activity. Mol. Endocrinol. 1997;11:2016–2024. doi: 10.1210/mend.11.13.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80a.Chen Y, Bender RA, Mews K, Adelmann G, Frotscher M, Baram TZ. The hippocampal CRH synpase: mismatched pre- and postsynaptic elements. Soc. Neurosci. Abs. 2002:867.9. [Google Scholar]
- 81.Smith BN, Dudek FE. Age-related epileptogenic effects of corticotropin-releasing hormone in the isolated CA1 region of rat hippocampal slices. J. Neurophysiol. 1994;72:2328–2333. doi: 10.1152/jn.1994.72.5.2328. [DOI] [PubMed] [Google Scholar]
- 82.Baram TZ, Hirsch E, Snead OC, Schultz L. Corticotropin-releasing hormone-induced seizures in infant rats originate in the amygdala. Ann. Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ehlers CL, Henriksen SJ, Wang M, Rivier J, Vale W, Bloom FE. Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Res. 1983;278:332–336. doi: 10.1016/0006-8993(83)90266-4. [DOI] [PubMed] [Google Scholar]
- 84.Marrosu F, Fratta W, Carrangiu P, Giagheddu M, Gessa GL. Localized epileptiform activity induced by murine CRF in rats. Epilepsia. 1988;29:369–373. doi: 10.1111/j.1528-1157.1988.tb03733.x. [DOI] [PubMed] [Google Scholar]
- 85.Baram TZ, Ribak CE. Peptide-induced infant status epilepticus causes neuronal death and synaptic reorganization. Neuroreport. 1995;6:277–280. doi: 10.1097/00001756-199501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sperber EF, Haas KZ, Stanton PK, Moshe SL. Resistance of the immature hippocampus to seizure-induced synaptic reorganization. Dev. Brain Res. 1991;60:88–93. doi: 10.1016/0165-3806(91)90158-f. [DOI] [PubMed] [Google Scholar]
- 87.Haas KZ, Sperber EF, Opanashuk LA, Stanton PK, Moshe SL. Resistance of immature hippocampus to morphologic and physiologic alterations following status epilepticus or kindling. Hippocampus. 2001;11:615–625. doi: 10.1002/hipo.1076. [DOI] [PubMed] [Google Scholar]
- 88.Pihoker C, Cain ST, Nemeroff CB. Postnatal development of regional binding of corticotropin-releasing factor and adenylate cyclase activity in the rat brain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1992;16:581–586. doi: 10.1016/0278-5846(92)90063-k. [DOI] [PubMed] [Google Scholar]
- 89.Brunson KL, Schultz L, Baram TZ. The in vivo proconvulsant effects of corticotropin releasing hormone in the developing rat are independent of ionotropic glutamate receptor activation. Dev. Brain Res. 1998;111:119–128. doi: 10.1016/s0165-3806(98)00130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lyons MK, Anderson RE, Meyer FB. Corticotropin releasing factor antagonist reduces ischemic hippocampal neuronal injury. Brain Res. 1991;545:339–342. doi: 10.1016/0006-8993(91)91310-w. [DOI] [PubMed] [Google Scholar]
- 91.Strijbos PJ, Relton JK, Rothwell NJ. Corticotropin-releasing factor antagonist inhibits neuronal damage induced by focal cerebral ischemia or activation of NMDA receptors in the rat brain. Brain Res. 1994;656:405–408. doi: 10.1016/0006-8993(94)91485-0. [DOI] [PubMed] [Google Scholar]
- 92.Maecker H, Desai A, Dash R, Rivier J, Vale W, Sapolsky R. Astressin, a novel and potent CRF antagonist, is neuroprotective in the hippocampus when administered after a seizure. Brain Res. 1997;744:166–170. doi: 10.1016/s0006-8993(96)01207-3. [DOI] [PubMed] [Google Scholar]
- 93.Pederson WA, Wan R, Zhang P, Mattson MP. Urocortin, but not urocortin II, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type I. J. Neurosci. 2002;22:404–412. doi: 10.1523/JNEUROSCI.22-02-00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J. Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 96.McEwen BS. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 97.Leverenz JB, Wilkinson CW, Wamble M, Corbin S, Grabber JE, Raskind MA, et al. Effect of chronic high-dose exogenous cortisol on hippocampal neuronal number in aged nonhuman primates. J. Neurosci. 1999;19:2356–2361. doi: 10.1523/JNEUROSCI.19-06-02356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shetty AK, Turner DA. Fetal hippocampal cells grafted to kainate-lesioned CA3 region of adult hippocampus suppress aberrant supragranular sprouting of host mossy fibers. Exp. Neurol. 1997;143:231–145. doi: 10.1006/exnr.1996.6363. [DOI] [PubMed] [Google Scholar]
- 99.Sapolsky RM. Stress, glucocorticoids, and damage to the nervous system: the current state of confusion. Stress. 1996;1:1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- 100.Dubé C, Brunson KL, Nehlig A, Baram TZ. Corticotropin releasing hormone activates specific neuronal circuits, as indicated by c-fos expression and glucose metabolism. J. Cereb. Blood Flow Metab. 2000;20:1414–1424. doi: 10.1097/00004647-200010000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Riviello P, de Rogalski Landrot I, Holmes GL. Lack of cell loss following recurrent neonatal seizures. Dev. Brain Res. 2002;135:101–104. doi: 10.1016/s0165-3806(02)00302-4. [DOI] [PubMed] [Google Scholar]