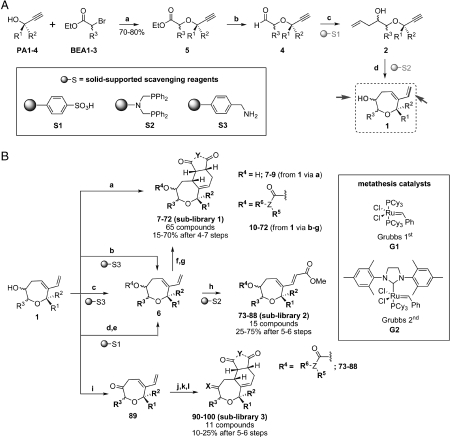
Scheme 2.
(A) General synthesis of the oxepane core. Diversification sites are highlighted (arrows). Building blocks, solid-supported scavengers, reagents, and conditions: (A) NaH, THF, 0 °C to room temperature (r.t.), 10 h; (B) DIBAL-H, Et2O, 78 °C, 20 min. then aqueous HCl (1 M), -78 °C to r.t.; (C) allylmagnesium chloride (2 M in THF), (+)-DIPCl or (−)-DIPCl, THF, -78 ° > C to r.t., 4 h, then solid-supported scavenger S1; (D) First generation Grubbs catalyst (10% mol), CH2Cl2, reflux, 18 h, then S2. (B) General synthesis of the oxepane collections. Building blocks, reagents, and conditions: (A) dienophile (D2), toluene, 70 °C, 3 h; (B) acyl chloride (AC1-8), pyridine, 6 h, r.t., then S3; (C) isocyanate (I1-4), pyridine, 6 h, r.t., then S3; (D) carbonyldiimidazole, CH2Cl2, r.t. overnight; (E) amine (A1-12), K2CO3, dimethylformamide (DMF), r.t. overnight, then S1; (F) dienophile (D1-5), toluene, 70 °C, 3 h; (G) 20% H2O in THF, 10 h, r.t.; (H) methyl acrylate, second generation Grubbs catalyst (15% mol), CH2Cl2, 18 h, reflux, then S2; (I) PCC, CH2Cl2, 10 h, r.t.; (J) dienophile (D1-5), toluene, 70 °C, 3 h; (K) hydroxylamine (HA1,2), EtOH/H2O (2∶1), r.t., 10 h; and (L) 20% H2O in THF, 10 h, r.t.