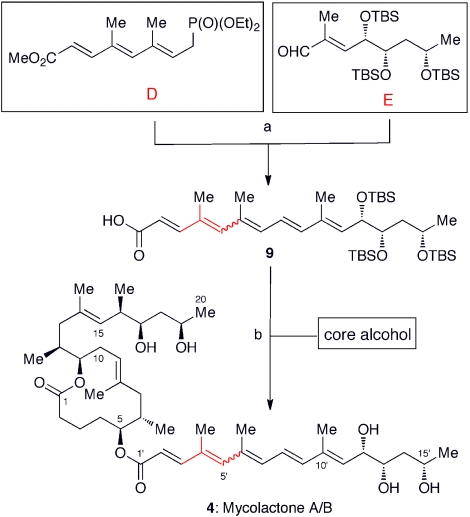
Fig. 10.
Convergent synthesis of unsaturated fatty acid side chain and completion of total synthesis. Aldehyde E was synthesized from commercially available, optically pure ethyl (S)-3-hydroxybutyrate. Sharpless asymmetric dihydroxylation was employed for incorporation of the C11′ and C12′ stereogenic centers of mycolactones A/B and D, whereas asymmetric Cr-mediated allylation was employed for incorporation of the 1,3-diols of mycolactones C, E, F, and dia-F. (A). Horner-Emmons reaction, followed by saponification. (B). Yamaguchi esterification, followed by TBS deprotection with TBAF. A wavy line indicates that this bond exists as a mixture of E- and Z-geometrical isomers.