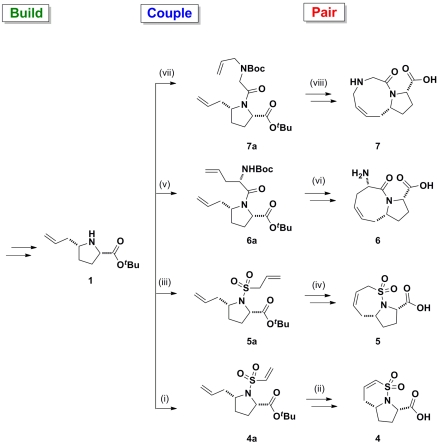
Fig. 2.
Application of a B/C/P approach starting from proline 1 yielding bicyclic compounds 4–7: (i) 2-chlorosulfonyl chloride, Et3N, CH2Cl2, 46%; (ii) Grubbs II, CH2Cl2, reflux, 80%; TFA; (iii) prop-2-ene-1-sulfonyl chloride, Et3N, CH2Cl2, 48%; (iv) Grubbs II, CH2Cl2, reflux, 90%; TFA; (v) (S)-N-Boc-allylglycine, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDCI), Oxyma, Et3N, CH2Cl2, 98%; (vi) Grubbs I, CH2Cl2, reflux, 87%; TFA; (vii) N-Boc-N-allylglycine, EDCI, Oxyma, Et3N, CH2Cl2, 97%; (viii) Grubbs I, CH2Cl2, reflux, 60%; TFA.